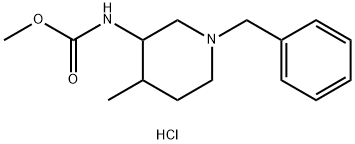
methyl (1-benzyl-4-methylpiperidin-3-yl)carbamate hydrochloride synthesis
- Product Name:methyl (1-benzyl-4-methylpiperidin-3-yl)carbamate hydrochloride
- CAS Number:1206824-67-0
- Molecular formula:C15H23ClN2O2
- Molecular Weight:298.81
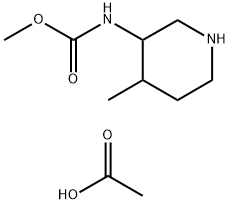
1206824-65-8
0 suppliers
inquiry

100-52-7
971 suppliers
$17.30/2g

1206824-67-0
18 suppliers
$255.00/250mg
Yield: 60%
Reaction Conditions:
Stage #1:methyl 4-methylpiperidin-3-ylcarbamate acetate;benzaldehyde in acetic acid;toluene at 20; for 2.5 h;
Stage #2: with sodium tris(acetoxy)borohydride in acetic acid;toluene at 20; for 18 h;
Stage #3: with hydrogenchloride in water;toluene at 80; for 2 h;
Steps:
1.c
c. To a stirred solution of methyl 4-methylpiperidin-3-ylcarbamate 5 (56.17 g, 326.59 mmol) and acetic acid (20 mL) in toluene (500 mL) was added benzaldehyde ( 51.98 g, 489.89 mmol) at 20 °C. The reaction was stirred at the same temperature for 2.5 h. The imine obtained was added to a stirred solution of sodium triacetoxyborohydride (103.82 g, 489.89 mmol) in toluene (300 mL) at 20 0C. The reaction was stirred for 18 h at the same temperature and pH was adjusted between 7.0 and 7.5 using aqueous sodium hydroxide (2N). The aqueous layer was separated and extracted with toluene (2 x 200 mL). The toluene layers were combined, added cone. HCl (70 mL) and heated to 80 °C for about 2 h. The solution was concentrated to dryness and the residue obtained was triturated with toluene. The solid obtained was collected by filtration and dried to afford methyl 1- benzyl-4-methylpiperidin-3-ylcarbamate hydrochloride 6 (36.5 g, 60% from 4) as a colorless crystalline solid.1H NMR (300 MHz, CDC13) δ 12.31 (s, IH, D2O exchangeable), 7.62 - 7.52 (m, 3H), 7.48 - 7.42 (m, 2H), 4.33 - 4.14 (m, 2H), 4.06 (d, J= 12.9, IH), 3.65 (s, 3H), 3.52 (d, J= 10.8, IH), 3.31 (d, J= 11.5, IH), 2.91 - 2.60 (m, 2H), 2.28 (d, J= 13.6, IH), 1.83 (s, IH), 1.66 (d, J= 15.1, IH), 0.97 (d, J = 6.5, 3H); MS (ES+): 263.2 (M +1).
References:
BIOCRYST PHARMACEUTICALS, INC.;BABU, Yarlagadda, S.;CHAND, Pooran;KOTIAN, Pravin, L.;KUMAR, V., Satish WO2010/14930, 2010, A2 Location in patent:Page/Page column 28; 31-32
3430-27-1
470 suppliers
$13.43/1gm:

1206824-67-0
18 suppliers
$255.00/250mg
694495-63-1
68 suppliers
$59.00/100mg

1206824-67-0
18 suppliers
$255.00/250mg