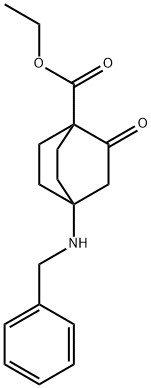
ethyl 4-(benzylamino)-2-oxobicyclo[2.2.2]octane-1-carboxylate synthesis
- Product Name:ethyl 4-(benzylamino)-2-oxobicyclo[2.2.2]octane-1-carboxylate
- CAS Number:1207166-75-3
- Molecular formula:C18H23NO3
- Molecular Weight:301.38
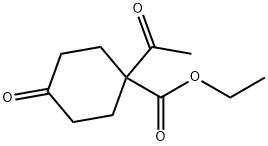
72653-21-5
29 suppliers
$175.00/100mg

100-46-9
465 suppliers
$5.00/5G
![ethyl 4-(benzylamino)-2-oxobicyclo[2.2.2]octane-1-carboxylate](/CAS/20180703/GIF/1207166-75-3.gif)
1207166-75-3
9 suppliers
inquiry
Yield:1207166-75-3 95%
Reaction Conditions:
Stage #1: ethyl 1-acetyl-4-oxocyclohexane-1-carboxylate;benzylaminewith toluene-4-sulfonic acid in toluene; for 7 h;Reflux;Dean-Stark conditions;
Stage #2: with hydrogenchloride in water;toluene at 20; for 0.5 h;
Stage #3: with sodium hydroxide in water;toluene;Product distribution / selectivity;
Steps:
3
Example 3 (Steps 1 and 2): 4-(Benzylamino)-2-oxobicyclo[2.2.2]octane-1-carboxylic acid ethyl ester Toluene (310 mL) was added to 1-acetyl-4-oxocyclohexyl carboxylic acid ethyl ester (31.0g, 146 mmol) and then the resulting mixture was stirred. To the mixture, there were added benzylamine (48.0 mL, 438 mmol) and p-toluenesulfonic acid monohydrate (251 mg, 1.32 mmol). The mixture was refluxed for 7 hours with a Dean-Stark apparatus under the dehydration conditions. The mixture was cooled to 20°C, a 3 mol/L hydrochloric acid solution (155 g) was added dropwise to the mixture and then the resulting mixture was stirred for 0.5 hours. To the mixture, there was added dropwise a 6 mol/L aqueous sodium hydroxide solution, followed by stirred for 10 minutes and the organic phase was separated. The resulting organic phase was washed twice with 155 g of a 18% aqueous ammonium chloride solution and further washed with 62 g of water. The organic phase was subjected to quantitative analysis (according to the LC technique) and as a result, the yield of the desired product was found to be 95% (the internal standard substance used was m-xylene).
References:
EP2322499,2011,A1 Location in patent:Page/Page column 17
17159-79-4
339 suppliers
$5.00/1g
![ethyl 4-(benzylamino)-2-oxobicyclo[2.2.2]octane-1-carboxylate](/CAS/20180703/GIF/1207166-75-3.gif)
1207166-75-3
9 suppliers
inquiry
![ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1489-97-0.gif)
1489-97-0
136 suppliers
$17.00/1g
![ethyl 4-(benzylamino)-2-oxobicyclo[2.2.2]octane-1-carboxylate](/CAS/20180703/GIF/1207166-75-3.gif)
1207166-75-3
9 suppliers
inquiry
1135619-58-7
2 suppliers
inquiry
![ethyl 4-(benzylamino)-2-oxobicyclo[2.2.2]octane-1-carboxylate](/CAS/20180703/GIF/1207166-75-3.gif)
1207166-75-3
9 suppliers
inquiry

141-97-9
781 suppliers
$5.00/25g
![ethyl 4-(benzylamino)-2-oxobicyclo[2.2.2]octane-1-carboxylate](/CAS/20180703/GIF/1207166-75-3.gif)
1207166-75-3
9 suppliers
inquiry