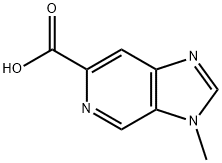
3-METHYL-3H-IMIDAZO[4,5-C]PYRIDINE-6-CARBOXYLIC ACID synthesis
- Product Name:3-METHYL-3H-IMIDAZO[4,5-C]PYRIDINE-6-CARBOXYLIC ACID
- CAS Number:1234014-38-0
- Molecular formula:C8H7N3O2
- Molecular Weight:177.16
![methyl 3-methyl-3H-imidazo[4,5-c]pyridine-6-carboxylate](/CAS/20200401/GIF/1234014-37-9.gif)
1234014-37-9
5 suppliers
inquiry
![3-METHYL-3H-IMIDAZO[4,5-C]PYRIDINE-6-CARBOXYLIC ACID](/CAS/20200331/GIF/1234014-38-0.gif)
1234014-38-0
5 suppliers
inquiry
Yield:1234014-38-0 90%
Reaction Conditions:
Stage #1: methyl 3-methyl-3H-imidazo[4,5-c]pyridine-6-carboxylatewith water;lithium hydroxide in tetrahydrofuran at 20; for 16 h;
Stage #2: with hydrogenchloride in water;
Steps:
E
To a stirred solution of 0.12 g (0.62 mmol) of methyl 3-methyl-3H-imidazo[4,5-c]pyridine-6- carboxylate E.4 in 2 mL of THF and 2 mL of water was added 52 mg (1.2 mmol) of lithium hydroxide at room temperature and the reaction mixture was stirred for 16 hr at room temperature. After completion of starting precursor (by TLC), volatiles were evaporated under reduced pressure. Resulting residue was diluted with water and washed with 10 mL of EtOAc. Aqueous layer was acidified using cone. HCl and evaporated under vacuum. The resulting residue was dried by co-distillation with toluene to afford 0.1 g (90 %) of 3-methyl-3H- imidazo[4,5-c]pyridine-6-carboxylic acid E.5 as light brown solid. 1H-NMR (200 MHz, DMSCw/6) δ 9.42 (s, 1H), 8.99 (s, 1H), 8.46 (s, 1H), 4.11 (s, 3H).
References:
WO2010/78408,2010,A1 Location in patent:Page/Page column 84; 85