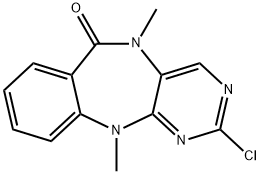
2-chloro-5,11-diMethyl-5H-benzo[e]pyriMido[5,4-b][1,4]diazepin-6(11H)-one synthesis
- Product Name:2-chloro-5,11-diMethyl-5H-benzo[e]pyriMido[5,4-b][1,4]diazepin-6(11H)-one
- CAS Number:1234479-75-4
- Molecular formula:C13H11ClN4O
- Molecular Weight:274.71
Yield:1234479-75-4 100%
Reaction Conditions:
with sodium hydride in N,N-dimethyl acetamide at -10 - 0; for 1.5 h;Inert atmosphere;
Steps:
2-chloro-5,11-dimethyl-5H-benzo[e]pyrimido[5,4-b][1,4] diazepin-6(11H)-one 11
Sodium hydride (0.24 g, 5.95 mmol) was added to a stirred suspension of 2-chloro-11-methyl-5H-benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one 10 (0.46 g, 1.75 mmol) and iodomethane (162 μL, 2.63 mmol) in N,N-dimethylacetamide (26.5 mL) at -10 °C. The solution was slowly warmed to 0 °C and stirred for 1.5 hours. When the reaction was complete, the solution was poured into ice-water and stirred on ice until a white precipitate formed. The product 11 was collected by filtration in quantitative yield and dried at the pump (0.49 g, 1.77 mmol, 100%).m.p. 125.3-125.6 oC; Rf 0.82 (1/10 (10% v/v aqueous ammonia in methanol/dichloromethane); IR (ZnSe cell, solid) vmax: 2920 (m, C-H), 1633 (m, lactam C=O), 1444 (s, C-H), 1401 (s, aromatic R3N), 1204 (aliphatic R3N), 939 (s, C-Cl), 752 (s, ring bending) cm-1; 1H NMR (400 MHz, d6-DMSO): δ 8.59 (1H, s, 6-CH), 7.70 (1H, d, 3J13-12 = 7.4 Hz, 13-CH), 7.55 (1H, dd, 3J11-12 ~ 3J11-10 = 7.2 Hz, 11-CH), 7.28 (1H, d, 3J10-11 = 8.0 Hz, 10-CH), 7.21 (1H, dd, 3J12-11 ~ 3J12-13 = 7.6 Hz, 12-CH), 3.42 (3H, s, 14-CH3), 3.34 (3H, s, 15-CH3) ppm; 13C NMR (100 MHz, d6-DMSO): δ 166.7 (7-C=O), 163.4 (4-C), 153.3 (2-C), 153.0 (6-CH), 148.2 (9-C), 133.1 (11-CH),132.0 (13-CH), 128.3 (5-C), 125.6 (8-C), 124.1 (12-CH), 118.5 (10-CH), 37.7 (14-CH3), 36.0 (15-CH3) ppm; LRMS (+ APCI) m/z: 275/277 ([M + H]+, 100/35%), 241 ([M-Cl- + 2H]+, 22%), 218 ([M-(H3CNC=O) + H]+, 12%); HRMS (+ APCI) m/z: 277.0664 (Cald. C13H1137ClN4O = 276.0592) ([M + H]+, 32%), 275.0699 (Calcd. C13H11ClN4O = 274.0621) ([M + H]+, 100%).
References:
Munoz, Lenka;Kavanagh, Madeline E.;Phoa, Athena F.;Heng, Benjamin;Dzamko, Nicolas;Chen, Ew-Jun;Doddareddy, Munikumar Reddy;Guillemin, Gilles J.;Kassiou, Michael [European Journal of Medicinal Chemistry,2015,vol. 95,p. 29 - 34] Location in patent:supporting information
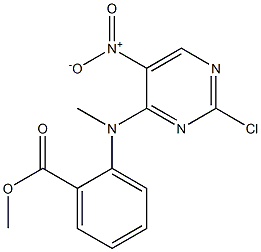
1234479-74-3
1 suppliers
inquiry
![2-chloro-5,11-diMethyl-5H-benzo[e]pyriMido[5,4-b][1,4]diazepin-6(11H)-one](/CAS/GIF/1234479-75-4.gif)
1234479-75-4
10 suppliers
inquiry
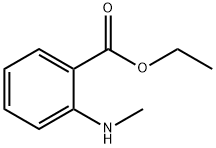
35472-56-1
50 suppliers
$31.00/100mg
![2-chloro-5,11-diMethyl-5H-benzo[e]pyriMido[5,4-b][1,4]diazepin-6(11H)-one](/CAS/GIF/1234479-75-4.gif)
1234479-75-4
10 suppliers
inquiry
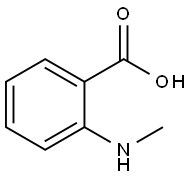
119-68-6
139 suppliers
$27.51/25g
![2-chloro-5,11-diMethyl-5H-benzo[e]pyriMido[5,4-b][1,4]diazepin-6(11H)-one](/CAS/GIF/1234479-75-4.gif)
1234479-75-4
10 suppliers
inquiry
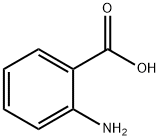
118-92-3
0 suppliers
$23.00/250g
![2-chloro-5,11-diMethyl-5H-benzo[e]pyriMido[5,4-b][1,4]diazepin-6(11H)-one](/CAS/GIF/1234479-75-4.gif)
1234479-75-4
10 suppliers
inquiry
![2-chloro-11-Methyl-5H-benzo[e]pyriMido[5,4-b][1,4]diazepin-6(11H)-one](/CAS/GIF/66427-86-9.gif)
