
Ethyl 2-(3-fluoro-4-methoxyphenyl)cyclopropanecarboxylate synthesis
- Product Name:Ethyl 2-(3-fluoro-4-methoxyphenyl)cyclopropanecarboxylate
- CAS Number:1234845-29-4
- Molecular formula:C13H15FO3
- Molecular Weight:238.25
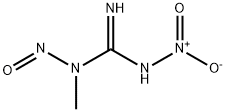
70-25-7
147 suppliers
$45.00/100mg
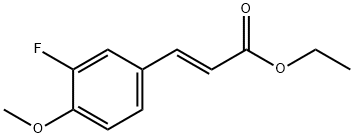
187872-44-2
2 suppliers
inquiry

1234845-29-4
12 suppliers
inquiry
Yield:1234845-29-4 83%
Reaction Conditions:
Stage #1: N-Methyl-N'-nitro-N-nitrosoguanidinewith water;potassium hydroxide in diethyl ether at 0; for 0.0333333 h;
Stage #2: ethyl (E)-3-(3-fluoro-4-methoxyphenyl)acrylate;palladium diacetate in diethyl ether at 0 - 5; for 4 h;
Stage #3: with acetic acid in diethyl ether;
Steps:
16.B
[0319] Step B: To a mixture of N-methyl-N'-nitro-N-nitrosoguanidine (TCI-America catalogue No. M0527, 3.7g on a dry weight basis, 25.0 mmol) in ether (50 mL) at 0 °C was added a cold solution of 25% aqueous ΚΟΗ (20mL). After stirring for 2 minutes, a portion of the yellow ethereal solution of the resulting diazomethane was added to a solution of the alkene (537) (l. lg, 5.0 mmol) in ether (25 mL) at 0 °C. A portion of palladium (II) acetate (0.112 g, 0.50 mmol) was added followed by an additional portion of diazomethane solution. This process was continued until all the diazomethane solution and palladium (II) acetate was added. The resulting mixture was stirred at 0-5 °C for 4 hours and acetic acid (6 drops) was added to quench any excess reagent. The resulting mixture was concentrated in vacuo to provide ethyl 2-(3-fluoro-4- methoxyphenyl)cyclopropane carboxylate (538) (0.990 g, 83.0%) as a yellow oil.
References:
WO2011/159297,2011,A1 Location in patent:Page/Page column 99-100
351-54-2
246 suppliers
$10.00/1g

1234845-29-4
12 suppliers
inquiry