
3-cyano-4-fluoro-N-(thiazol-2-yl)benzenesulfonaMide synthesis
- Product Name:3-cyano-4-fluoro-N-(thiazol-2-yl)benzenesulfonaMide
- CAS Number:1235406-28-6
- Molecular formula:C10H6FN3O2S2
- Molecular Weight:283.3
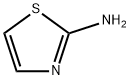
96-50-4
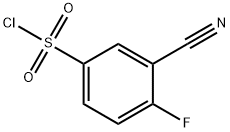
351003-23-1

1235406-28-6
General procedure for the synthesis of 3-cyano-4-fluoro-N-(thiazol-2-yl)benzenesulfonamide from 2-aminothiazole and 3-cyano-4-fluorobenzenesulfonyl chloride: 3-cyano-4-fluorobenzenesulfonyl chloride (10 g, 45.53 mmol) was added batchwise to a mixed solution of dichloromethane (50 mL) and pyridine (18.4 mL) containing 2-aminothiazole (5 g, 50.13 mmol) , 228 mmol) in a mixed solution. The reaction mixture was gradually warmed up to room temperature. After 1 hour of reaction, a precipitate was observed to be generated. The mixture was stirred continuously for 18 hours at room temperature. Subsequently, the mixture was sonicated for 2.5 hours until the solids were completely dissolved, after which stirring was continued for 18 hours at room temperature. Upon completion of the reaction, the mixture was concentrated under reduced pressure and azeotropically dried with toluene (2 x 100 mL) to remove residual water. The dried residue was carefully diluted with 1 M aqueous hydrochloric acid and stirred at room temperature for 1 hour to induce precipitate formation. The brown solid product was collected by filtration and purified by milling with dichloromethane to afford finally 3-cyano-4-fluoro-N-(thiazol-2-yl)benzenesulfonamide as a brown solid (7.8 g, 60% yield). The product was characterized by 1H NMR (d6-DMSO): δ 6.90 (m, 1H), 7.30 (m, 1H), 7.65 (t, 1H), 8.15 (m, 1H), 8.30 (m, 1H), 12.90 (br s, 1H).The results of the LCMS analysis were as follows: retention time Rt = 2.18 min, mass m/z 284 [MH]+, 282 [MH]-.

96-50-4
555 suppliers
$5.00/5g

351003-23-1
107 suppliers
$20.00/1g

1235406-28-6
27 suppliers
inquiry
Yield:1235406-28-6 60%
Reaction Conditions:
Stage #1: 2-thiazolylamine;3-cyano-4-fluorobenzene-1-sulfonyl chloridewith pyridine in dichloromethane at 0 - 20;Sonographic reaction;
Stage #2: with hydrogenchloride in water at 20; for 1 h;
Steps:
32
3-Cyano-4-fluorobenzenesulfonyl chloride (10 g, 45.53 mmol) was added portion wise to a solution of 2-aminothiazole (5 g, 50.13 mmol) in dichloromethane (50 mL) and pyridine (18.4 mL, 228 mmol) at 0° C. The reaction mixture was allowed to warm to room temperature. After 1 hour a precipitate was observed. The mixture was stirred for 18 hours at room temperature. The mixture was sonicated for 2.5 hours until the solid had dissolved, then left to stir at room temperature for 18 hours. The reaction mixture was then concentrated in vacuo and azeotropically dried with toluene (2×100 mL). The residue was diluted carefully with a 1M aqueous solution of hydrogen chloride and stirred for 1 hour at room temperature whereupon a precipitate formed. The brown solid was collected by filtration and triturated with dichloromethane to afford the title compound as a brown solid (7.8 g, 60%).1HNMR (d6-DMSO): δ 6.90 (m, 1H), 7.30 (m, 1H), 7.65 (t, 1H), 8.15 (m, 1H), 8.30 (m, 1H), 12.90 (br s, 1H).LCMS Rt=2.18 minutes MS m/z 284 [MH]+, 282 [MH]-
References:
US2012/10182,2012,A1 Location in patent:Page/Page column 39