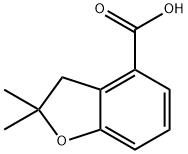
2,2-Dimethyl-2,3-dihydrobenzofuran-4-carboxylic acid synthesis
- Product Name:2,2-Dimethyl-2,3-dihydrobenzofuran-4-carboxylic acid
- CAS Number:123656-35-9
- Molecular formula:C11H12O3
- Molecular Weight:192.21
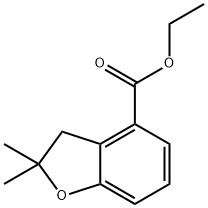
209256-58-6

123656-35-9
Ethyl 2,2-dimethyl-2,3-dihydrobenzofuran-4-carboxylate (10 g, 45 mmol) was used as starting material, which was mixed with a solution of sodium hydroxide (16.3 ml, 10N, 163 mmol) in ethanol (50 ml) and heated to reflux for 2 hours. Upon completion of the reaction, the mixture was concentrated under vacuum and subsequently diluted with water and acidified with 12N hydrochloric acid to the appropriate pH. The precipitate produced after acidification was collected by filtration and dried in air to give 2,2-dimethyl-2,3-dihydrobenzofuran-4-carboxylic acid (8.6 g, 100% yield).

209256-58-6
0 suppliers
inquiry

123656-35-9
18 suppliers
inquiry
Yield:123656-35-9 100%
Reaction Conditions:
Stage #1: ethyl 2,2-dimethyl-2,3-dihydro-benzofuran-4-carboxylatewith sodium hydroxide in ethanol; for 2 h;Heating / reflux;
Stage #2: with hydrogenchloride in water;
Steps:
6.3
Ethyl 2,2-dimethyl-2,3-dihydro-benzofuran-4-carboxylic acid (10 g, 45 mmol) was heated to reflux with sodium hydroxide (16.3 ml of 10 N, 163 mmol) in ethanol (50 ml) for 2 hr. The mixture was concentrated in vacuo, diluted with water and made acidic with 12 N HCl. The precipitate was filtered and air dried (8.6 g, 100%).
References:
EP1027043,2004,B1 Location in patent:Page/Page column 20

929301-86-0
8 suppliers
inquiry

123656-35-9
18 suppliers
inquiry
19438-10-9
258 suppliers
$6.00/5g

123656-35-9
18 suppliers
inquiry
![Benzoic acid, 3-[(2-methyl-2-propen-1-yl)oxy]-, methyl ester](/CAS/20200611/GIF/136954-98-8.gif)
136954-98-8
1 suppliers
inquiry

123656-35-9
18 suppliers
inquiry
136955-00-5
1 suppliers
inquiry

123656-35-9
18 suppliers
inquiry