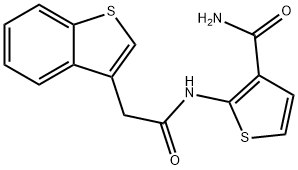
2-(2-(benzo[b]thiophen-3-yl)acetaMido)thiophene-3-carboxaMide synthesis
- Product Name:2-(2-(benzo[b]thiophen-3-yl)acetaMido)thiophene-3-carboxaMide
- CAS Number:1246072-89-8
- Molecular formula:C15H12N2O2S2
- Molecular Weight:316.4
![BENZO[B]THIOPHENE-3-ACETIC ACID](/CAS/GIF/1131-09-5.gif)
1131-09-5
79 suppliers
$29.39/500mg
14080-51-4
135 suppliers
$8.00/250mg
![2-(2-(benzo[b]thiophen-3-yl)acetaMido)thiophene-3-carboxaMide](/CAS/GIF/1246072-89-8.gif)
1246072-89-8
1 suppliers
inquiry
Yield:1246072-89-8 31%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20; for 18 h;
Steps:
Synthesis of 4,5-dimethyl-2-(2-(naphthalen-1-yl)acetamido)thiophene-3-carboxamide (1, BI-90G8) :
General procedure: A mixture 2-amino-4,5-dimethylthiophene-3-carboxamide (340 mg, 2 mmol), 2-(1-naphthalen-1-yl)acetic acid (372 mg, 2 mol), EDC (458 mg, 2.4 mmol), HOBt (324 mg, 2.4 mmol), DIEA ( 1.1 mL, 6 mmol) in DMF (10 mL) was stirred at room temperature for 18 h. The reaction mixture was poured into 100 mL of water then extracted with ethyl acetate (150 mL). The organic layer was washed with saturated NaHCO3 solution (3 x 50 mL), brine (3 x 50 mL), water (3 x 50 mL) respectively. The ethyl acetate layer was dried over MgSO4 and concentrated. The residue was chromatographed over silica gel (30 to 40% ethyl acetate in haxane) to afford compound 1 (277 mg, 41%).
References:
De, Surya K.;Barile, Elisa;Chen, Vida;Stebbins, John L.;Cellitti, Jason F.;MacHleidt, Thomas;Carlson, Coby B.;Yang, Li;Dahl, Russell;Pellecchia, Maurizio [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 8,p. 2582 - 2588] Location in patent:supporting information; experimental part