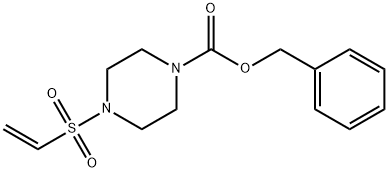
1-Piperazinecarboxylic acid, 4-(ethenylsulfonyl)-, phenylmethyl ester synthesis
- Product Name:1-Piperazinecarboxylic acid, 4-(ethenylsulfonyl)-, phenylmethyl ester
- CAS Number:1246203-64-4
- Molecular formula:C14H18N2O4S
- Molecular Weight:310.37
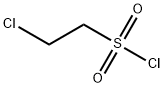
1622-32-8
196 suppliers
$31.00/25g
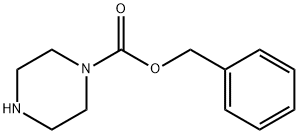
31166-44-6
216 suppliers
$5.00/1g

1246203-64-4
4 suppliers
inquiry
Yield:1246203-64-4 43%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at -15 - 0; for 0.75 h;
Steps:
83
A mixture of 11 g (50 mmol) of benzyl 1-piperazinecarboxylate and 16.1 g (125 mmol) of DIPEA in 200 mL CH2Cl2 was cooled to -15° C. and 6.3 mL (9.8 g, 60 mmol) of 2-chloroethanesulfonyl chloride was added slowly over 15 min. The mixture was allowed to warm to 0° C. over 30 min and water was then added. The organic layer was separated and washed successively with dil. HCl and aq. NaHCO3. After drying, the solvent was removed and the residue was chromatographed on silica, eluting with CH2Cl2/EtOAc 95:5, to give an oil, which was recrystallized from CH2Cl2/hexanes to give 6.64 g (43% yield) of benzyl 4-(vinylsulfonyl)-1-piperazinecarboxylate: mp (CH2Cl2/hexanes) 85-87° C.; 1H NMR (CDCl3) δ 7.39-7.30 (m, 5H), 6.40 (dd, J=16.6, 9.8 Hz, 1H), 6.25 (d, J=16.6 Hz, 1H), 6.06 (d, J=9.8 Hz, 1H), 5.14 (s, 2H), 3.61 (m, 4H), 3.14 (m, 4H); Anal. Calcd. for C14H18N2O4S: C, 54.2; H, 5.85; N, 9.0; Found: C, 54.1; H, 5.7; N, 9.1%.
References:
US2010/249099,2010,A1 Location in patent:Page/Page column 87