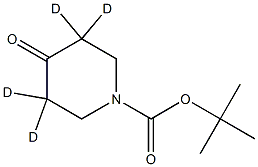
tert-butyl 4-oxopiperidine-1-carboxylate-3,3,5,5-d4 synthesis
- Product Name:tert-butyl 4-oxopiperidine-1-carboxylate-3,3,5,5-d4
- CAS Number:1246342-66-4
- Molecular formula:C10H13D4NO3
- Molecular Weight:203.2715

79099-07-3
0 suppliers
$5.00/5g

1246342-66-4
15 suppliers
inquiry
Yield:1246342-66-4 86%
Reaction Conditions:
with chloroform-d1;1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine at 20; under 760.051 Torr;
Steps:
Preparation of tert-Butyl 3,3,5,5-tetradeutero-4-oxopiperidine-l-carboxylate (23).
Preparation of tert-Butyl 3,3,5,5-tetradeutero-4-oxopiperidine-l-carboxylate (23). l-Boc-4-piperidone (10 g, 50.2 mmol) was dissolved in CDC13 (100 mL). 1,5,7- Triazabicyclo[4.4.0]dec-5-ene (0.5 g) was added as a single portion and the solution was stirred at ambient temperature and pressure overnight. The deuterium enrichment was assayed by 1H NMR and the reaction was deemed complete when resonances assigned to the protons alpha to the carbonyl were no longer visible by 1H NMR. The reaction was neutralized with aqueous hydrochloric acid (1M) and the product extracted with ethyl acetate. The combined organics were dried over sodium sulfate, filtered and concentrated to give tert-butyl 3,3,5,5-tetradeutero-4-oxopiperidine-l-carboxylate 23 as a colorless oil (8.72 g, 43.0 mmol, 86% yield, >99% D4) 1H NMR (400 MHz, CDC13) δ: 3.71 (s, 4H), 1.47 (s, 9H).
References:
WO2014/81816,2014,A1 Location in patent:Paragraph 00165