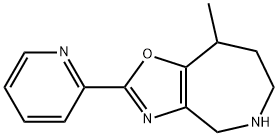
4H-Oxazolo[4,5-c]azepine, 5,6,7,8-tetrahydro-8-Methyl-2-(2-pyridinyl)- synthesis
- Product Name:4H-Oxazolo[4,5-c]azepine, 5,6,7,8-tetrahydro-8-Methyl-2-(2-pyridinyl)-
- CAS Number:1247885-34-2
- Molecular formula:C13H15N3O
- Molecular Weight:229.28
![4H-Oxazolo[4,5-c]azepine, 5,6,7,8-tetrahydro-8-methyl-5-[(4-methylphenyl)sulfonyl]-2-(2-pyridinyl)-](/CAS/20210305/GIF/1247885-33-1.gif)
1247885-33-1
0 suppliers
inquiry
![4H-Oxazolo[4,5-c]azepine, 5,6,7,8-tetrahydro-8-Methyl-2-(2-pyridinyl)-](/CAS/GIF/1247885-34-2.gif)
1247885-34-2
4 suppliers
inquiry
Yield:1247885-34-2 40%
Reaction Conditions:
Stage #1: 8-methyl-2-(pyridin-2-yl)-5-tosyl-5,6,7,8-tetrahydro-4H-oxazolo[4,5-c]azepinewith hydrogen bromide in water at 100; for 1 h;
Stage #2: with sodium hydroxide in water; pH=13;
Steps:
215.k
A solution of 8-methyl-2-(pyridin-2-yl)-5-tosyl-5,6,7,8-tetrahydro-4H- oxazolo[4,5-c]azepine (76 mg, 0.2 mmol) in 48% aqueous HBr (1 mL) was heated to 1000C and stirred for 1 h. The reaction was cooled to room temperature and extracted with tert-butyl methyl ether. The aqueous layer was adjusted to pH 13 with sodium hydroxide and extracted with DCM. The combined organic layers were dried over sodium sulfate, filtered and evaporated to give the title compound as a yellow oil. (18 mg, 40%); LC/MS: m/e = 230 (M+H)+.
References:
WO2010/114971,2010,A1 Location in patent:Page/Page column 234