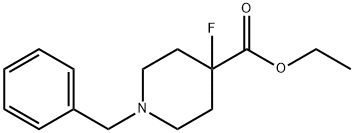
4-Piperidinecarboxylic acid, 4-fluoro-1-(phenylMethyl)-, ethyl ester synthesis
- Product Name:4-Piperidinecarboxylic acid, 4-fluoro-1-(phenylMethyl)-, ethyl ester
- CAS Number:1250443-08-3
- Molecular formula:C15H20FNO2
- Molecular Weight:265.32
24228-40-8
248 suppliers
$9.59/250mg

1250443-08-3
1 suppliers
inquiry
Yield:1250443-08-3 66%
Reaction Conditions:
Stage #1: N-benzyl isonipecotic acid ethyl esterwith n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at -40 - -10; for 1.16667 h;
Stage #2: with N-fluorobis(benzenesulfon)imide in tetrahydrofuran at -78 - -65; for 1.33333 h;
Steps:
X.1
General Procedure X: Nucleophilic fluorination at the a-position of an esterA flask is charged with a base (such as LDA, LiHMDS, NaHMDS, NaOEt, NaOMe, KOtBu, or NaNH2, preferably LDA) (1 to 3 equiv, preferably 1.5 equiv) in an organic solvent (such as THF, 1 ,4-dioxane, Et20 or DMF, preferably THF). The temperature of the mixture is adjusted to about -78 to -30 °C (preferably about -40 °C) and a solution of an ester (preferably 1 equiv) in an organic solvent (such as 1,4-dioxane, THF, Et20, or DMF preferably THF) is added slowly so as to maintain the temperature of the mixture within about +/- 10 °C. The mixture is stirred at about -40 to 0 °C (preferably about -15 to -10 °C) for about 30 min to 2 h (preferably about 1 h), and is then cooled to about -78 to -40 °C (preferably about -78 °C). To the mixture is slowly added a solution of A^-fluoro-A^-(phenylsulfonyl)benzenesulfonamide (1-2 equiv, preferably 1.05 equiv) in an organic solvent (such as 1,4-dioxane, THF, Et20 or DMF, preferably THF) to maintain the temperature of the mixture within about +/- 15 °C. The resulting mixture is stirred at about -78 to 0 °C (preferably about -78 °C) for about 30 min to 4 h (preferably about 1 h). The mixture is poured into ice water and extracted with an organic solvent (such as DCM or EtOAc). The combined extracts are optionally washed with water and/or brine, dried over anhydrous Na2S04 or MgS04, filtered or decanted and concentrated under reduced pressure. The crude material is optionally purified by silica gel chromatography. Illustration of General Procedure XPreparation No.X.l: Ethyl l-benzyl-4-fluoropiperidine-4-carboxylateA flask was charged with DIEA (8.64 mL, 60.6 mmol) and THF (100 mL). The mixture was cooled to about -78 °C followed by the addition of ?-BuLi (24.3 mL, 60.6 mmol) over about 5 min. The mixture was warmed to about 0 °C and stirred for about 30 min and then cooled to about -40 °C. To the mixture was added a solution of ethyl l-benzylpiperidine-4-carboxylate (10.0 g, 40.4 mmol, Oakwood) in THF (75 mL) over about 10 min such that the solution is kept between -40 to -30 °C. The mixture was stirred for about 1 h at about -10 °C before being cooled to about -78 °C. A solution of A^-fluoro-A^-(phenylsulfonyl)benzenesulfonamide (14.0 g, 44.5 mmol) in THF (75 mL) was added over about 20 min while keeping the mixture between about - 65 and -78 °C. The mixture was then stirred for about 1 h at about -78 °C. The mixture was poured into a separatory funnel containing ice water (30 mL) and EtOAc (30 mL) was added. The aqueous layer was extracted with EtOAc (3 x 25 mL) and the combined organic extracts were washed with brine, dried over anhydrous Na2S04, filtered and concentrated under reduced pressure. The resulting mixture was adsorbed onto silica gel and purified by flash silica gel chromatography eluting with hexanes/EtOAc (10:1) to afford ethyl l-benzyl-4-fluoropiperidine-4- carboxylate (7.13 g, 66 %): LC/MS (Table 2, Method h) Rt = 2.21 min; MS m/z: 266 (M+H)+.
References:
WO2012/48222,2012,A1 Location in patent:Page/Page column 185-186