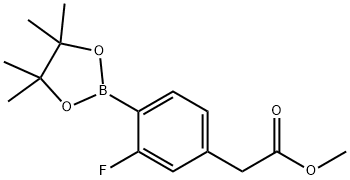
2-Fluoro-4-(MethoxycarbonylMethyl)benzeneboronic acid pinacol ester, 96% synthesis
- Product Name:2-Fluoro-4-(MethoxycarbonylMethyl)benzeneboronic acid pinacol ester, 96%
- CAS Number:1259022-70-2
- Molecular formula:C15H20BFO4
- Molecular Weight:294.13
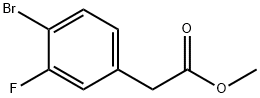
942282-41-9
38 suppliers
$163.00/250mg

73183-34-3
594 suppliers
$6.00/5g

1259022-70-2
18 suppliers
$148.05/250mg
Yield:1259022-70-2 81%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 110; for 16 h;Inert atmosphere;Temperature;
Steps:
32.6 Methyl 2-(3-fluoro-4-(4,4,5 5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetate
To a mixture of methyl 2-(4-bromo-3-fluorophenyl)acetate (5 g, 20.24 mmol) in 1,4-dioxane (10 mL) was added 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (5.65 g, 22.26 mmol), KOAc (3.97 g, 40.5 mmol) and PdCl2(dppf) (1.481 g, 2.024 mmol). The resulting suspension was stirred at 110 °C for 16 h under nitrogen. The mixture was then filtered and concentrated. The crude material was purified by silica column chromatography (PE/EA = 5: 1). All fractions found to contain product by TLC (PE/EA = 5: 1, Rf = 0.6) were combined and concentrated to yield a yellow oil of methyl 2-(3-fluoro-4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenyl)acetate (6 g, 16.32 mmol, 81% yield): lH NMR (400 MHz, MeOH- 4) δ 7.66-7.58 (m, 1H), 7.07 (d, J = 7.6 Hz, 1H), 6.98 (d, J= 10.4 Hz, 1H), 3.67 (s, 5H), 1.33 (s, 12H); ES-LCMS m/z 295.1 (M+H).
References:
WO2014/141187,2014,A1 Location in patent:Page/Page column 130; 79
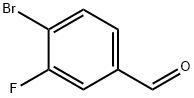
133059-43-5
240 suppliers
$6.00/1g

1259022-70-2
18 suppliers
$148.05/250mg
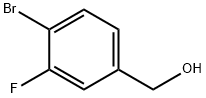
222978-01-0
113 suppliers
$11.00/250mg

1259022-70-2
18 suppliers
$148.05/250mg
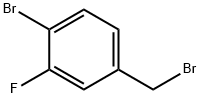
127425-73-4
141 suppliers
$7.00/250mg

1259022-70-2
18 suppliers
$148.05/250mg
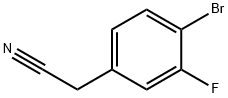
499983-13-0
98 suppliers
inquiry

1259022-70-2
18 suppliers
$148.05/250mg