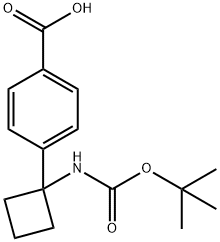
4-(1-(tert-butoxycarbonylaMino)cyclobutyl)benzoic acid synthesis
- Product Name:4-(1-(tert-butoxycarbonylaMino)cyclobutyl)benzoic acid
- CAS Number:1259223-99-8
- Molecular formula:C16H21NO4
- Molecular Weight:291.34

1259223-99-8
22 suppliers
inquiry

18107-18-1
210 suppliers
$33.00/5g
![Benzoic acid, 4-[1-[[(1,1-dimethylethoxy)carbonyl]amino]cyclobutyl]-, methyl ester](/CAS/20180629/GIF/1338243-68-7.gif)
1338243-68-7
3 suppliers
inquiry
Yield:1338243-68-7 93%
Reaction Conditions:
with methanol in hexanes;dichloromethane at 0 - 20; for 1 h;
Steps:
5.3.1
Example 3Synthesis of tert-butyl (1-(4-(hydrazinecarbonyl)phenyl)cyclobutyl)carbamateStep 1. Synthesis of methyl 4-(1-((tert-butoxycarbonyl)amino)cyclobutyl)benzoateTo a solution of 4-(1-((tert-butoxycarbonyl)amino)cyclobutyl)benzoic acid (15 g, 52 mmol) in dichloromethane (200 mL) and methanol (50 mL), cooled at 0° C., was added in a dropwise manner a 2 M solution of diazomethyl trimethylsilane in hexanes (28 mL, 57 mmol). The reaction mixture was stirred at room temperature for one hour before the addition, at 0° C., of acetic acid (10 mL). The mixture was washed with saturated aqueous sodium bicarbonate solution until the aqueous phase remained basic. The organic phase was washed with brine, dried over sodium sulfate, filtered and concentrated in vacuo, yielding the methyl 4-(1-((tert-butoxycarbonyl)amino)cyclobutyl)benzoate as a white crystalline solid (14.8 g, 93%). M.p.=90° C.; 400 MHz 1H NMR (DMSO-d6) δ: 7.91 (d, J=7.6 Hz, 2H), 7.74 (s, 1H), 7.50 (d, J=8.0 Hz, 2H), 3.84 (s, 3H), 2.38 (br.s, 4H), 2.1-1.9 (m, 1H), 1.85-1.7 (m, 1H), 1.32 (br.s, 9H); LCMS [M-tBu+H]: 250.
References:
US2011/245236,2011,A1 Location in patent:Page/Page column 94

1259223-99-8
22 suppliers
inquiry
1313883-38-3
1 suppliers
inquiry

1259223-99-8
22 suppliers
inquiry
![Carbamic acid, N-[1-[4-[2-(2-amino-3-pyridinyl)-5-chloro-3H-imidazo[4,5-b]pyridin-3-yl]phenyl]cyclobutyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1313883-39-4.gif)
1313883-39-4
1 suppliers
inquiry

1259223-99-8
22 suppliers
inquiry
![tert-butyl (1-(4-(2-(2-aminopyridin-3-yl)-5-phenyl-3H-imidazo[4,5-b]pyridin-3-yl)phenyl)cyclobutyl)carbamate](/CAS/20180601/GIF/1313881-69-4.gif)
1313881-69-4
2 suppliers
inquiry

1259223-99-8
22 suppliers
inquiry
![Carbamic acid, N-[1-[4-[5-[3-[4-(acetylmethylamino)-1-piperidinyl]phenyl]-2-(2-amino-3-pyridinyl)-3H-imidazo[4,5-b]pyridin-3-yl]phenyl]cyclobutyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1416775-98-8.gif)
1416775-98-8
0 suppliers
inquiry