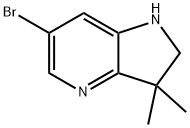
6-bromo-3,3-dimethyl-2,3-dihydro-1H-pyrrolo[3,2-b]pyridine synthesis
- Product Name:6-bromo-3,3-dimethyl-2,3-dihydro-1H-pyrrolo[3,2-b]pyridine
- CAS Number:1259512-11-2
- Molecular formula:C9H11BrN2
- Molecular Weight:227.1
![6-bromo-3,3-dimethyl-1H-pyrrolo[3,2-b]pyridin-2(3H)-one](/CAS/20180527/GIF/1190862-33-9.gif)
1190862-33-9
38 suppliers
inquiry
![6-bromo-3,3-dimethyl-2,3-dihydro-1H-pyrrolo[3,2-b]pyridine](/CAS/20180703/GIF/1259512-11-2.gif)
1259512-11-2
33 suppliers
inquiry
Yield:1259512-11-2 100%
Reaction Conditions:
with sodium tetrahydroborate;boron trifluoride diethyl etherate in tetrahydrofuran at 0 - 20; for 6 h;Inert atmosphere;
Steps:
8.5 Step 5) 6-bromo-3,3-dimethyl-2,3-dihydro-1H-pyrrolo[3,2-b]pyridine
To a solution of 6-bromo-3,3-dimethyl-lH-pyrrolo[3,2-b]pyridin-2(3H)-one (1.21 g, 5 mmol) in THF (100 mL) was added NaBH4 (0.95 g, 25 mmol), followed by the dropwise addition of BF3-Et20 (47%, 9.5 mL, 35 mmol) at 0 °C under 2 atmosphere. The reaction was stirred at rt for 6 h, then quenched with saturated aqueous NH4C1 (25 mL). The resulted mixture was continued to stirred at rt overnight, then diluted with H20 (100 mL) and extracted with EtOAc (100 mL x 3). The combined organic phases were dried over anhydrous Na2S04 and concentrated in vacuo. The residue was purified by a silica gel column chromatography (100% DCM) to give the title compound as a yellow solid (1.46 g, 100%). MS (ESI, pos. ion) m/z: 227 [M + H]+.
References:
WO2014/89324,2014,A1 Location in patent:Paragraph 0218

1403901-48-3
0 suppliers
inquiry
![6-bromo-3,3-dimethyl-2,3-dihydro-1H-pyrrolo[3,2-b]pyridine](/CAS/20180703/GIF/1259512-11-2.gif)
1259512-11-2
33 suppliers
inquiry
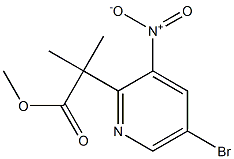
1259512-10-1
6 suppliers
inquiry
![6-bromo-3,3-dimethyl-2,3-dihydro-1H-pyrrolo[3,2-b]pyridine](/CAS/20180703/GIF/1259512-11-2.gif)
1259512-11-2
33 suppliers
inquiry
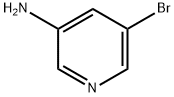
13535-01-8
384 suppliers
$3.00/1g
![6-bromo-3,3-dimethyl-2,3-dihydro-1H-pyrrolo[3,2-b]pyridine](/CAS/20180703/GIF/1259512-11-2.gif)
1259512-11-2
33 suppliers
inquiry