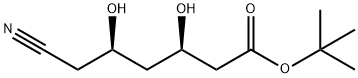
(3R,5R)-6-Cyano-3,5-dihydroxy-hexanoic Acid tert-Butyl Ester synthesis
- Product Name:(3R,5R)-6-Cyano-3,5-dihydroxy-hexanoic Acid tert-Butyl Ester
- CAS Number:125971-93-9
- Molecular formula:C11H19NO4
- Molecular Weight:229.27
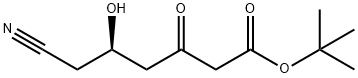
125988-01-4
69 suppliers
inquiry

125971-93-9
58 suppliers
inquiry
Yield: 90%
Reaction Conditions:
with D-glucose in water at 28; for 24 - 48 h;Microbiological reaction;
Steps:
1
Mucor cirdnelloides MTCC 5187 was maintained on Malt Extract Agar (MEA) plates prepared in sterilized MEA (5%) in distilled water.For growth in liquid medium a loopful of microbial cells was aseptically transferred from an agar plate to a 250 mL baffled conical flask containing 50 ml of medium having the following composition (per liter) - K2 HPO4 (1.9 g), NaH2 PO4.2H2 O (2.02 g) (NH4)2 SO4 (1.8 g), MgSO4 7H2 O (0.2 g), FeCI3 (0.97 mg) and trace elements solution (1 ml). Trace elements solution consisted of (per liter) CuSO45H2 O (0.02 g), MnSO44H2 O (0.1 g), ZnSO4.7H2 O (0.1 g) and CaCO3 (1.8 g). The above minimal medium was supplemented with 0.2% (w/v) yeast extract and 2.25% (w/v) glucose.The microorganism was grown at 280C on an orbital shaker at 220 rpm for 24-48 hours. Microbial cells were harvested by centrifuging at 7000 rpm for EPO
References:
BIOCON LIMITED WO2006/131933, 2006, A1 Location in patent:Page/Page column 5-6
143-33-9
0 suppliers
$10.00/1g
154026-93-4
53 suppliers
$215.00/10g

125971-93-9
58 suppliers
inquiry
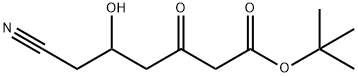
848644-99-5
1 suppliers
inquiry
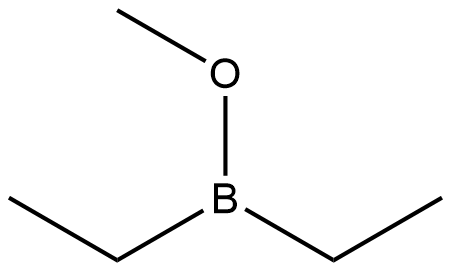
7397-46-8
183 suppliers
$33.70/25ml

125971-93-9
58 suppliers
inquiry
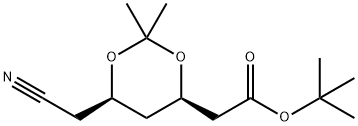
125971-94-0
354 suppliers
$12.00/1g
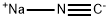
773837-37-9
0 suppliers
inquiry

154026-93-4
53 suppliers
$215.00/10g

125971-93-9
58 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

125971-93-9
58 suppliers
inquiry