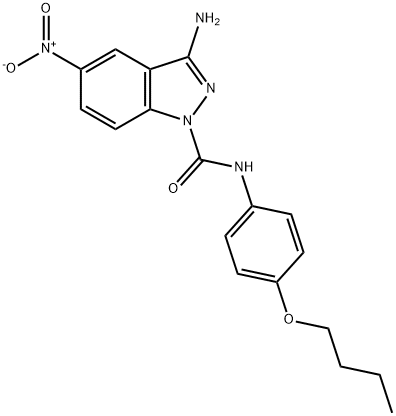
3-Amino-N-(4-butoxyphenyl)-5-nitro-1H-indazole-1-carboxamide synthesis
- Product Name:3-Amino-N-(4-butoxyphenyl)-5-nitro-1H-indazole-1-carboxamide
- CAS Number:1263320-07-5
- Molecular formula:C18H19N5O4
- Molecular Weight:369.37
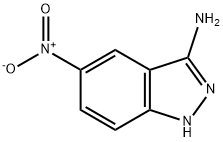
41339-17-7
133 suppliers
$15.00/250mg
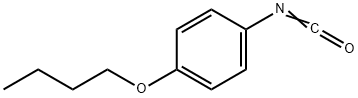
28439-86-3
36 suppliers
$32.75/1g

1263320-07-5
5 suppliers
inquiry
Yield:1263320-07-5 72%
Reaction Conditions:
in tetrahydrofuran at 20; for 24 h;
Steps:
5.1.2. General procedure for the preparation of compounds 1c-h,l,m
General procedure: A solution of equimolar amounts of indazole derivative 2c,e [16], g [17] or indazole 2l [18] and phenyl isocyanate derivative 3a,b [18] (7 mmol) in THF (20 mL) was stirred at room temperature for 24 h. Then, the solvent was removed under reduced pressure and the solid residue was crystallized from a suitable solvent or purified as described below.
References:
Maggio, Benedetta;Raimondi, Maria Valeria;Raffa, Demetrio;Plescia, Fabiana;Cascioferro, Stella;Plescia, Salvatore;Tolomeo, Manlio;Di Cristina, Antonietta;Pipitone, Rosaria Maria;Grimaudo, Stefania;Daidone, Giuseppe [European Journal of Medicinal Chemistry,2011,vol. 46,# 1,p. 168 - 174] Location in patent:experimental part