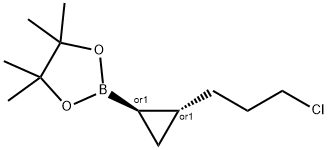
2-(2-(3-chloropropyl)cyclopropyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-(2-(3-chloropropyl)cyclopropyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:126726-63-4
- Molecular formula:C12H22BClO2
- Molecular Weight:244.57

75-11-6
350 suppliers
$10.00/5g
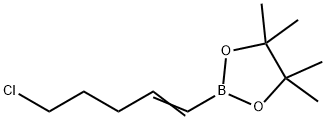
154820-95-8
14 suppliers
$637.00/25g

126726-63-4
12 suppliers
inquiry
Yield:126726-63-4 194 g
Reaction Conditions:
Stage #1: diiodomethanewith diethylzinc;trifluoroacetic acid in n-heptane;dichloromethane at 3; for 0.5 h;Inert atmosphere;
Stage #2: 2-(5-chloropent-1-en-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in n-heptane;dichloromethane at 20; for 20 h;Inert atmosphere;
Steps:
1 Preparation of 2-[2-(3-Chloro-propyl)-cyclopropyl]-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane (Compound 3)
Compound 3 was produced as follows:
To a 5 L flask equipped with a nitrogen inlet, mechanical stirrer, dropping funnel and thermocouple under N2 was added 800 mL dichloromethane and 800 mL of a 1 M diethylzinc solution in heptane (0.8 mol, 1.07 equiv).
The solution was cooled with an ice bath to an internal temperature of 3° C.
To the flask was then added from the dropping funnel a solution of 57.6 mL trifluoroacetic acid (0.748 mol, 1.0 equiv) in 200 mL dichloromethane over 1 hour, keeping the internal temperature below 10° C.
The resulting suspension was stirred for 30 min at 3° C.
To the flask was then added 72.4 mL diiodomethane (0.897 mol, 1.2 equiv) in a single portion.
After stirring at 3° C. for 30 min, 172 mL of 2 (0.748 mol, 1.0 equiv) was added to the solution in a single portion.
The flask was then allowed to warm to room temperature and a white precipitate began to form.
After 3 hours, GC analysis indicated the reaction was at 90% conversion.
The suspension was aged for an additional 17 hours or until complete consumption of 2 is observed.
At that point, 800 mL of 1 M HCl (0.8 mol, 1.07 equiv) was added and a +5 exotherm was observed.
The biphasic mixture was stirred for 30 min to dissolve the precipitated solids and the organic layer was separated.
Extraction of the aqueous layer with 200 mL dichloromethane, washing of the combined organic layers with 500 mL brine and concentration in vacuo gave 194 g of 3 as a yellow oil (74 wt % in DCM).
1H NMR (400 MHz, CDCl3) δ 3.59 (t, 2H, J=6.7 Hz), 1.90 (pent, 2H, J=7.1 Hz), 1.49 (sext, 1H, J=7.0 Hz), 1.36 (sext, 1H, J=7.0 Hz), 1.23 (s, 12H), 0.93 (m, 1H), 0.71 (m, 1H), 0.44 (m, 1H), -0.35 (dt, 1H, J=9.4, 5.7 Hz); 13C NMR (100 MHz, CDCl3) δ 82.82, 44.74, 32.67, 32.22, 24.64, 17.22, 11.24, 0.5 (bs); GC: HP1 (30 m*0.32 mm; 0.25 μm), 25 psi, 200° C. front inlet.
5 min 50° C., ramp 25° C./min to 250° C. then hold for 4 min, tr(2)=9.78 min, tr(3)=10.08 min.
References:
US9238604,2016,B2 Location in patent:Page/Page column 30; 31
14267-92-6
263 suppliers
$10.00/1g
25015-63-8
217 suppliers
$22.39/5G

126726-63-4
12 suppliers
inquiry