
Spiro[1-azabicyclo[2.2.2]octane-3,5'(4'H)-oxazole], 2'-(1-methyl-1H-indol-3-yl)-, (-)- synthesis
- Product Name:Spiro[1-azabicyclo[2.2.2]octane-3,5'(4'H)-oxazole], 2'-(1-methyl-1H-indol-3-yl)-, (-)-
- CAS Number:128200-30-6
- Molecular formula:C18H21N3O
- Molecular Weight:295.38
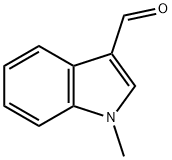
19012-03-4
211 suppliers
$6.00/1g
![Spiro[1-azabicyclo[2.2.2]octane-3,5'(4'H)-oxazole], 2'-(1-methyl-1H-indol-3-yl)-, (-)-](/CAS/20210305/GIF/128200-30-6.gif)
128200-30-6
0 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 3 steps
1: (H3N)2HOP4, AcOH, PrNO2
2: 72 percent / methanol / 24 h / Ambient temperature
3: 66 percent / HCl / methanol / 1.) 12 h under reflux, 2.) 12 h reflux in saturated methanolic HCl
References:
Swain, Christopher J.;Kneen, Clare;Herbert, Richard;Baker, Raymond [Journal of the Chemical Society. Perkin transactions I,1990,# 11,p. 3183 - 3186]
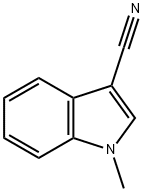
24662-37-1
66 suppliers
$33.39/250mg
![Spiro[1-azabicyclo[2.2.2]octane-3,5'(4'H)-oxazole], 2'-(1-methyl-1H-indol-3-yl)-, (-)-](/CAS/20210305/GIF/128200-30-6.gif)
128200-30-6
0 suppliers
inquiry

![Boron, [3-(aminomethyl)-1-azabicyclo[2.2.2]octan-3-ol-κN1]trihydro-, (T-4)-](/CAS/20211123/GIF/128653-87-2.gif)