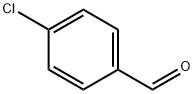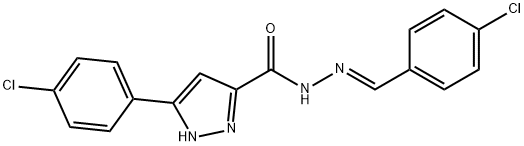
(E)-N-(4-chlorobenzylidene)-3-(4-chlorophenyl)-1H-pyrazole-5-carbohydrazide synthesis
- Product Name:(E)-N-(4-chlorobenzylidene)-3-(4-chlorophenyl)-1H-pyrazole-5-carbohydrazide
- CAS Number:1284275-59-7
- Molecular formula:C17H12Cl2N4O
- Molecular Weight:359.21
Yield:1284275-59-7 60%
Reaction Conditions:
with acetic acid in methanol; for 6 h;Reflux;
Steps:
General procedure for the synthesis of hydrazones (4a-j)
General procedure: The hydrazones were prepared by condensation of hydrazide (10.0 mmol) and the appropriate aromatic aldehyde (10.5 mmol), in 55 ml of mixture of glacial CH3COOH:CH3OH (5:50) under reflux for 6 h. After cooling, the crude product was collected by filtration, washed and crystallized from hot ethanol to give a hydrazone (4a-j).
References:
Pathak, Ravindra B.;Chovatia;Parekh [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 15,p. 5129 - 5133] Location in patent:supporting information; experimental part
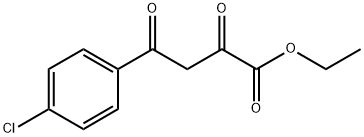
5814-38-0
48 suppliers
$28.60/10MG

1284275-59-7
5 suppliers
inquiry
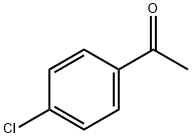
99-91-2
424 suppliers
$10.00/25g

1284275-59-7
5 suppliers
inquiry