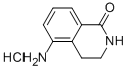
5-AMINO-3,4-DIHYDROISOQUINOLIN-1(2H)-ONE HYDROCHLORIDE synthesis
- Product Name:5-AMINO-3,4-DIHYDROISOQUINOLIN-1(2H)-ONE HYDROCHLORIDE
- CAS Number:129075-52-1
- Molecular formula:C9H11ClN2O
- Molecular Weight:198.65
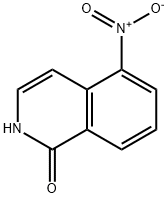
82827-08-5
44 suppliers
inquiry

129075-52-1
15 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride;Pd-C in ethanol;
Steps:
IX 5-Amino-3,4-dihydro-1(2H)-isoquinolinone, monohydrochloride
EXAMPLE IX 5-Amino-3,4-dihydro-1(2H)-isoquinolinone, monohydrochloride A mixture of 19.0 g (119 mmol) of 5-nitroisoquinolinone in 1.7 liters of ethanol and 1.0 g of 5% Pd-C was hydrogenated at room temperature for 2.1 hours (three atmospheres). Then 4.0 g of 20% Pd-C was added and hydrogenation was continued. After 19.8 hours an additional 2.0 g of 20% Pd-C was added. At the end of 40 hours the reaction was filtered and concentrated. The residue was recrystallized from ethanol/hexane to give 13.4 g (70%) of product suitable for further reaction. An analytical sample was obtained by dissolving a sample in ethanol, followed by treatment with a saturated solution of ethanol/HCl, cooling and collecting the resulting hydrochloride salt; mp 284°-302°.
References:
US5177075,1993,A