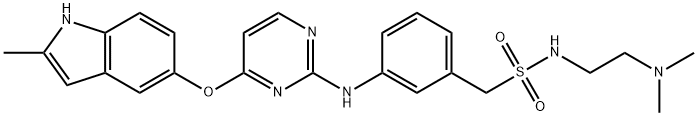
Surufatinib synthesis
- Product Name:Surufatinib
- CAS Number:1308672-74-3
- Molecular formula:C24H28N6O3S
- Molecular Weight:480.58

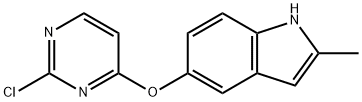
1071105-16-2
4 suppliers
inquiry
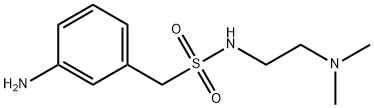
1094797-87-1
18 suppliers
inquiry

1308672-74-3
69 suppliers
inquiry
Yield:-
Reaction Conditions:
with toluene-4-sulfonic acid in N,N-dimethyl-formamide at 55 - 65;
Steps:
1.2
To a 10 L three-necked round bottom flask, equipped with amechanical stirrer and a thermometer, were added Compound 3 (1 .05 Kg), 1 -(3- aminophenyl)-/V-(2-(dimethylamino)ethyl)methanesulfonamide (Compound 4, 1.06 Kg), p-toluenesulfonic acid (0.86 Kg), and /VJV-dimethylformamide (5.25 L). The reaction mixture was carefully warmed to a temperature ranging from 55 to 65 °C, and stirred at this temperature for 16-20 hours. After the completion of the reaction, the reaction mixture was cooled to room temperature, and was transferred portion-wise to a solution of 5% aqueous potassium carbonate.When the addition was complete, the obtained slurry was stirred for another 1 -2 hours. Crude product was collected by filtration, and the wet filter cake was transferred to a 200 L reactor.[0116] To the reactor, toluene (104 Kg) was added, and the suspension was heated to reflux to remove water by a Dean-Stark trap. After the removal of water, the solution was concentrated to a final volume of 30-40 L under reduced pressure, cooled to 15-20 °C. The product was collected and dried to afford the title product (Compound of Formula A, 1.07 Kg). This material can then be used to produce novel forms of the compound of Formula A, such as Form I and/or Form II.
References:
Location in patent:Page/Page column 27