
Sodium hydroxy(4-(methoxycarbonyl)bicyclo-[2.2.2]octan-1-yl)methanesulfonate synthesis
- Product Name:Sodium hydroxy(4-(methoxycarbonyl)bicyclo-[2.2.2]octan-1-yl)methanesulfonate
- CAS Number:1308728-99-5
- Molecular formula:C11H19NaO6S
- Molecular Weight:302.32
![4-Formyl-bicyclo[2.2.2]octane-1-carboxylic acid methyl ester](/CAS/20180808/GIF/94994-25-9.gif)
94994-25-9
53 suppliers
inquiry
![Sodium hydroxy(4-(methoxycarbonyl)bicyclo-[2.2.2]octan-1-yl)methanesulfonate](/CAS/GIF/1308728-99-5.gif)
1308728-99-5
8 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydrogensulfite in 2-methyltetrahydrofuran at 20;Industry scale;
Steps:
3
Preparation of 13 from Acid Chloride 11: 9.4 Kg of 11 was dissolved in 9.4 liters of 2-MeTHF and subsequently added to a mixture of 0.94 kg of Pd/BaS04 and 5.18 Kg (1.05eq) of collidine in 49.4 liters of 2- MeTHF under nitrogen. The reaction was allowed to stir under hydrogen pressure at about 30°C. The mixture was then cooled to 20°C and filtered. The collidine was removed by washing the resulting mixture with acid, e.g., aqueous HC1. The organic layer was further washed with aqueous Na2C03 followed by addition of 98 Kg of 39%> aqueous NaHS03 (9 eq) at about 20°C to convert 11 to crystalline 13. The heterogeneous mixture was filtered, washed with MTBE and dried in vacuo to give 9.77 Kg of 13 in 93% molar yield from 11.
References:
WO2011/63268,2011,A2 Location in patent:Page/Page column 37-38
![BICYCLO[2.2.2]OCTANE-1,4-DICARBOXYLIC ACID HEMIMETHYL ESTER](/CAS/GIF/18720-35-9.gif)
18720-35-9
245 suppliers
$19.00/1g
![Sodium hydroxy(4-(methoxycarbonyl)bicyclo-[2.2.2]octan-1-yl)methanesulfonate](/CAS/GIF/1308728-99-5.gif)
1308728-99-5
8 suppliers
inquiry
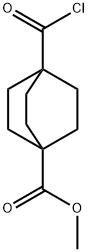
110371-29-4
2 suppliers
inquiry
![Sodium hydroxy(4-(methoxycarbonyl)bicyclo-[2.2.2]octan-1-yl)methanesulfonate](/CAS/GIF/1308728-99-5.gif)
1308728-99-5
8 suppliers
inquiry