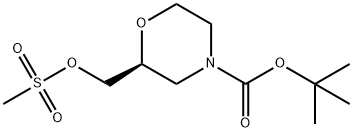
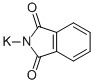
2-(1,3-Dioxo-1,3-dihydro-isoindol-2-ylMethyl)-Morpholine-4-carboxylic acid tert-butyl ester synthesis
- Product Name:2-(1,3-Dioxo-1,3-dihydro-isoindol-2-ylMethyl)-Morpholine-4-carboxylic acid tert-butyl ester
- CAS Number:1308849-93-5
- Molecular formula:C18H22N2O5
- Molecular Weight:346.38
Yield:1308849-93-5 88%
Reaction Conditions:
in N,N-dimethyl-formamide at 110 - 115; for 16 h;
Steps:
4.B
Part B. Preparation of (R)-tert-butyl 2-((l,3-dioxoisoindolin-2- yl)methyl)morpholine-4-caPotassium phthalimide (28.4 g 0.15 mol) was added to a solution of (5)-tert-butyl 2- ((methylsulfonyloxy)methyl)morpholine-4-carboxylate (37.7 g, 0.13 mmol) in DMF (256 mL). The resulting mixture was stirred at 1 10-115°C for 16 hr, and then cooled to room temperature and poured into water (500 mL). The aqueous layer was extracted with CH2CI2 (3 x 250 mL). The combined organic layers were then washed with brine, dried over MgS04, filtered, and concentrated under reduced pressure. The residue was diluted in hexanes (200 mL) and then stirred vigorously while adding slowly Et20 (100 mL). The oil turned into a solid, which was filtered on a Buchner funnel. The filter cake was washed with hexanes and dried under reduced pressure. The mother liquor was concentrated, and the crude residue purified by silica gel flash chromatography (10-60% EtOAc in hexanes) to produce a second crop of solid. Both solids were combined to provide the title compound (38.9 g, 88%). ? NMR (300 MHz, CDC13) δ ppm 1.45 (s, 9 H), 2.68-2.82 (m, 1 H), 2.91-3.04 (m, 1 H), 3.44 (dt, J= 1 1.45, 2.81 Hz, 1 H), 3.63-3.79 (m, 3 H), 3.82-4.03 (m, 3 H), 7.70-7.78 (m, 2 H), 7.84-7.88 (m, 2 H).
References:
WO2011/62550,2011,A1 Location in patent:Page/Page column 29-30
135065-76-8
185 suppliers
$16.00/1g

1308849-93-5
6 suppliers
inquiry