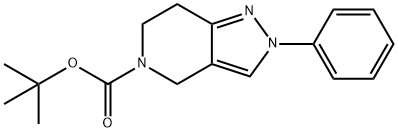
tert-butyl 2-phenyl-6,7-dihydro-2H-pyrazolo[4,3-c]pyridine-5(4H)-carboxylate synthesis
- Product Name:tert-butyl 2-phenyl-6,7-dihydro-2H-pyrazolo[4,3-c]pyridine-5(4H)-carboxylate
- CAS Number:1310796-20-3
- Molecular formula:C17H21N3O2
- Molecular Weight:299.37
![tert-butyl (3Z)-3-[(dimethylamino)methylidene]-4-oxopiperidine-1-carboxylate](/CAS/20180601/GIF/1310796-19-0.gif)
1310796-19-0
7 suppliers
inquiry
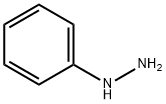
100-63-0
383 suppliers
$10.00/1g
![5-Boc-1-Phenyl-1,4,6,7-tetrahydropyrazolo[4,3-c]pyridine](/CAS2/GIF/1075729-08-6.gif)
1075729-08-6
20 suppliers
$90.00/50mg
![tert-butyl 2-phenyl-6,7-dihydro-2H-pyrazolo[4,3-c]pyridine-5(4H)-carboxylate](/CAS/20130318/GIF/CB12602171.gif)
1310796-20-3
8 suppliers
$960.00/5G
Yield:-
Reaction Conditions:
in methanol; for 3 h;Reflux;
Steps:
7.I.b; 7.II.b
Step b) 2-Phenyl-2,4,6,7-tetrahydropyrazolo[4,3-c]pyridine-5-carboxylic acid tert-butyl ester (b-I) and 1-phenyl-1,4,6,7-tetrahydropyrazolo[4,3-c]pyridine-5-carboxylic acid tert-butyl ester (b-II)):The product of step a) (2.4 g) is dissolved in 27 ml of methanol. 1.24 g of phenylhydrazine are added and the mixture is refluxed for 3 hours. Evaporation is carried out under vacuum and column-purification is carried out by flash chromatography using a Biotage column, elution being carried out with a mixture of ethyl acetate and cyclohexane. 1.8 g of an oily material are isolated.
References:
US2012/245149,2012,A1 Location in patent:Page/Page column 13

79099-07-3
0 suppliers
$5.00/5g
![tert-butyl 2-phenyl-6,7-dihydro-2H-pyrazolo[4,3-c]pyridine-5(4H)-carboxylate](/CAS/20130318/GIF/CB12602171.gif)
1310796-20-3
8 suppliers
$960.00/5G

79099-07-3
0 suppliers
$5.00/5g
![5-Boc-1-Phenyl-1,4,6,7-tetrahydropyrazolo[4,3-c]pyridine](/CAS2/GIF/1075729-08-6.gif)
1075729-08-6
20 suppliers
$90.00/50mg
![tert-butyl 2-phenyl-6,7-dihydro-2H-pyrazolo[4,3-c]pyridine-5(4H)-carboxylate](/CAS/20130318/GIF/CB12602171.gif)
1310796-20-3
8 suppliers
$960.00/5G