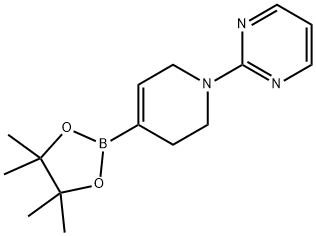
2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5,6-dihydropyridin-1(2H)-yl)pyrimidine synthesis
- Product Name:2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-5,6-dihydropyridin-1(2H)-yl)pyrimidine
- CAS Number:1312479-75-6
- Molecular formula:C15H22BN3O2
- Molecular Weight:287.17
Yield: 75.4%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane at 80;Inert atmosphere;
Steps:
67.A
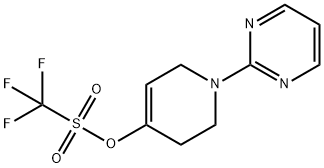
To a flask containing 1 -pyrimidin-2-yl-1 ,2,3,6-tetrahydropyridin-4-yltrifluoromethanesulfonate (300 mg, 0.970 mmol) (may be prepared according to the procedures in PCT Int Appl 2006124897), bis(pinacolato)diboron (296 mg, 1.16 mmol), potassium acetate (288 mg, 2.91 mmol), 1 , 1 '-bis-(diphenylphosphino)ferrocene (16 mg, 0.029 mmol) and [1 , 1 '-bis-(diphenylphosphino)ferrocene]-dichloropalladium (II) dichloromethane complex (71 mg, 0.097 mmol) was added deoxygenated 1 ,4-dioxane (5.0 ml). The reaction was placed under vacuum and opened to nitrogen three times and then heated at 80°C under nitrogen overnight. The reaction was cooled and purified directly by chromatography on silica gel with 6:1 heptane-ethyl acetate to give 2-[4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)-3,6-dihydropyridin-1 (2H)-yl]pyrimidine as a yellow solid (210 mg, 75.4%). 1H NMR (400 MHz, CHLOROFORM-d) δ ppm 1 .29 (s, 12 H) 2.25 - 2.44 (m, 2 H) 3.89 (t, J=5.66 Hz, 2 H) 4.24 - 4.29 (m, 1 H) 6.42 - 6.54 (m, 1 H) 6.58 - 6.69 (m, 1 H) 8.21 - 8.40 (m, 2 H). LCMS: 288.4 M+1.
References:
PFIZER INC.;BROWN, Matthew Frank;CHE, Ye;MARFAT, Anthony;MELNICK, Michael Joseph;MONTGOMERY, Justin Ian;REILLY, Usa WO2011/73845, 2011, A1 Location in patent:Page/Page column 137-138