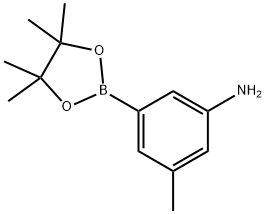
3-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline synthesis
- Product Name:3-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline
- CAS Number:1312535-18-4
- Molecular formula:C13H20BNO2
- Molecular Weight:233.11
74586-53-1
118 suppliers
$15.00/2g

73183-34-3
555 suppliers
$6.00/5g

1312535-18-4
11 suppliers
inquiry
Yield:1312535-18-4 88%
Reaction Conditions:
with anhydrous potassium acetate;dicyclohexyl(2’,4’,6’-triisopropyl-[ 1,1’-bi-phenyl]-2-yl)phosphane;tris-(dibenzylideneacetone)dipalladium(0) in 1,4-dioxane at 20 - 80; for 4 h;Inert atmosphere;
Steps:
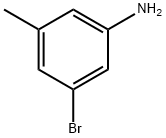
Dioxane (720 mL) in a 1 L three-necked round bottom flask was degassed for 30 min. 3-Bromo-5-methylaniline (60g, 193 mmol), (bispinacolato)diboron (96 g, 377 mmol), potassium acetate (42.7 g, 435 mmol), X-Phos (8.3 g, 17.41 mmol) and Pd2dba3 (3.99 g, 4.35 mmol) were added to the degassed solvent under N2(g). After Stirling for 10 min at room temperature, the system was heated to an internal temperature of 80 °C. After ca. 4 hours, the heating mantle was removed and replaced with an ice water bath. The reaction mixture was cooled to 30 °C, and was then filtered through a pad of celite (washing with 500 mL of MTBE). This was transferred to a 4 L separatory funnel containing 500 mL pH 8 phosphate buffer, 500 mL brine, and an additional 500 mL of MTBE. The layers were cut and the organic washed with 1 L of a 1 : 1 mixture of brine and water. The aqueous layers were combined and were sequentially back extracted with a second 500 mL portion of MTBE. The combined organics were treated with 100 g of MgS04 and the resulting mixture stirred for 20 min. This was then filtered and concentrated in vacuo. The resultant residue was purified by flash chromatography (Biotage, 0-25% ethyl acetate in hexanes) to yield 3-methyl-5-(4,4,5,5-tetramethyl-l ,3,2-dioxaborolan-2-yl)aniline (66 g, 255 mmol, 88%) as a light orange solid. MS APCI: [M + H]+ /z 234.2.
References:
WO2011/75560,2011,A1 Location in patent:Page/Page column 30