
tert-butyl 4-aMino-4-(2-hydroxyethyl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-aMino-4-(2-hydroxyethyl)piperidine-1-carboxylate
- CAS Number:1312784-58-9
- Molecular formula:C12H24N2O3
- Molecular Weight:244.33
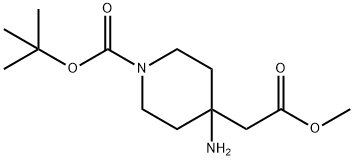
362703-57-9
58 suppliers
$213.40/250mg

1312784-58-9
1 suppliers
inquiry
Yield:1312784-58-9 66%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 1.33333 h;Inert atmosphere;
Steps:
128.A Step A:
tert-butyl 4-amino-4-(2-methoxy-2-oxoethyl)piperidine-1-carboxylate (3.0 g, 11 mmol) was dissolved in tetrahydrofuran (30 mL, 11 mmol) in a 100 mL reaction flask charged with a stir bar. The solution was cooled to 0°C and placed under nitrogen. Lithium aluminum hydride (19 mL, 19 mmol) (1M in THF, anhydrous) was added dropwise over 20 minutes via addition funnel. The reaction was stirred from 0°C to RT for 1 hour. The reaction was cooled to 0°C and quenched with water (.72 μL) followed by 15% NaOH (.72 μL) then water (2.1 mL). The mixture was stirred at RT for 30 minutes. The crude solution was filtered through celite. The solids were washed with EtOAc and the filtrate was condensed to afford tert-butyl 4-amino-4-(2-hydroxyethyl)piperidine-1-carboxylate (1.8 g, 66% yield). m/z (esi/APCI) M+1 = 245.2.
References:
WO2020/81848,2020,A1 Location in patent:Paragraph 00526

79099-07-3
0 suppliers
$5.00/5g

1312784-58-9
1 suppliers
inquiry