
6-methyl-2-pyridin-2-yl-1,3,6,2-dioxazaborocane-4,8-dione synthesis
- Product Name:6-methyl-2-pyridin-2-yl-1,3,6,2-dioxazaborocane-4,8-dione
- CAS Number:1313758-49-4
- Molecular formula:C10H11BN2O4
- Molecular Weight:234.0163

1313758-49-4
1 suppliers
inquiry
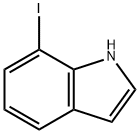
89976-15-8
43 suppliers
$90.00/250mg
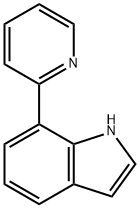
117908-12-0
0 suppliers
inquiry
Yield:117908-12-0 76%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);copper diacetate;potassium carbonate;tricyclohexylphosphine in N,N-dimethyl-formamide;isopropyl alcohol at 100; for 24 h;Autoclave;Inert atmosphere;Suzuki Coupling;
Steps:
3 Synthesis of ligand 7-PyIn
351.0mgof 6-methyl-2- (pyridine-2-yl) -1,3,6,2- dioxazaborocane -4,8-dione (1.5 mmol),243.0mg of 7 - iodo indole (1 mmol), 13.7mg of [Pd2 (dba) 3] (0.015 mmol), 16.8mgof PCy3 (0.06 mmol), 90.8mg of Cu (OAc) 2 and 691mg of K2CO3 were weighed to anautoclave of 70ml and covered with inert gas, and 40ml N, N- dimethyl methaneamide and 10ml isopropanol were added. The autoclave was sealed and the contentswere stirred for 24 hours at 100 . The reaction mixturewas imported to dichloromethane 30 ml, and washed 3x with H2O, then the aqueousphase was extracted 3x for each time with dichloromethane 30 ml, the organicphase wasdried over MgSO4, filtered and concentrated in vacuum. The product was isolated using a silica gelcolumn (dichloromethane: hexane 4: 6, Rf = 0.56). 76% yield.
References:
JP2016/501830,2016,A Location in patent:Paragraph 0104; 0105