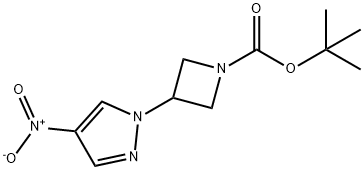
tert-butyl3-(4-nitro-1H-pyrazol-1-yl)azetidine-1-carboxylate synthesis
- Product Name:tert-butyl3-(4-nitro-1H-pyrazol-1-yl)azetidine-1-carboxylate
- CAS Number:1314987-79-5
- Molecular formula:C11H16N4O4
- Molecular Weight:268.27
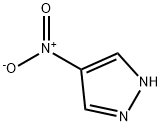
2075-46-9
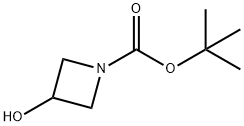
141699-55-0

1314987-79-5
General procedure for the synthesis of tert-butyl 3-(4-nitro-1H-pyrazol-1-yl)azetidine-1-carboxylate from 4-nitropyrazole and N-Boc-3-hydroxyazetidine: DIAD (3.92 mL, 19.9 mmol, 1.5 eq.) was slowly added dropwise under nitrogen protection to a stirring mixture of 4-nitropyrazole (1.5 g, 13.27 mmol), N-Boc-3-hydroxyazetidine, and triphenylphosphine (5.22 g, 19.9 mmol, 1 eq.). 13.27 mmol), N-Boc-3-hydroxyazetidine (2.3 g, 13.27 mmol, 1 eq.) and triphenylphosphine (5.22 g, 19.9 mmol, 1.5 eq.) in a solution of THF (30 mL) and the reaction mixture was placed in an ice bath. The reaction was stirred at 0 °C for 10 min, then gradually warmed to room temperature and continued stirring for 16 h. The reaction was carried out at 0 °C for 10 min, then gradually warmed to room temperature and continued stirring. After completion of the reaction, the reaction mixture was diluted with ethyl acetate (100 mL) and washed sequentially with water (40 mL) and brine (30 mL x 2). The organic layers were combined, dried over anhydrous sodium sulfate and concentrated. The crude product was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 1/10) to afford tert-butyl 3-(4-nitro-1H-pyrazol-1-yl)azetidine-1-carboxylate as a light yellow solid (3 g, 85% yield).ESI-MS (M + H-56): 213.1.1.1H NMR (400 MHz, CDCl3) δ: 8.28 (s 1H), 8.16 (s, 1H), 5.07-5.04 (m, 1H), 4.44-4.40 (m, 2H), 4.34-4.30 (m, 2H), 1.47 (s, 9H).

2075-46-9
332 suppliers
$6.00/5g
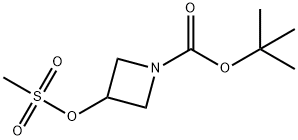
141699-58-3
113 suppliers
$6.00/250mg

1314987-79-5
30 suppliers
inquiry
Yield:1314987-79-5 93%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 90; for 12 h;
Steps:
30.1 Step 1: Synthesis of tert-butyl-3-(4-nitro-1H-pyrazol-1-yl)azetidin-1-carboxylate
4-Nitro-1H-pyrazole (2.9 g, 25.77 mmol) was dissolved in DMF (30 ml). Cs2CO3 (17.6 g, 2.0 eq.) and tert-butyl-3-(methylsulfonyloxy)azetidin-1-carboxylate (6.8 g, 1.05 eq., Intermediate 21) were added thereto, followed by stirring thereof at 90°C for 12 hours. Water was added thereto to terminate the reaction. It was extracted three times with ethyl acetate, dried over MgSO4, and concentrated. It was subjected to column chromatography (MeOH:MC = 1:40) to obtain 6.45 g of the target compound in yellow color (yield: 93%). MS (ESI) m/z : 268.9 [M+H]+; 1H NMR (300 MHz, CDCl3) δ 8.30 (s, 1H), 8.14 (s, 1H), 5.09-5.03 (m, 1H), 4.44-4.29 (m, 4H), 1.46 (s, 9H).
References:
EP3722298,2020,A1 Location in patent:Paragraph 0147

2075-46-9
332 suppliers
$6.00/5g

141699-55-0
398 suppliers
$6.00/1g

1314987-79-5
30 suppliers
inquiry

2075-46-9
332 suppliers
$6.00/5g
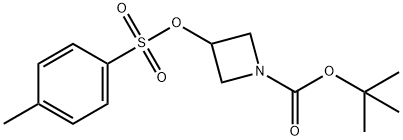
605655-08-1
37 suppliers
$98.00/250mg

1314987-79-5
30 suppliers
inquiry

141699-55-0
398 suppliers
$6.00/1g

1314987-79-5
30 suppliers
inquiry
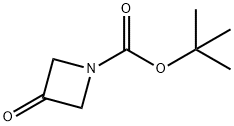
398489-26-4
445 suppliers
$9.00/5g

1314987-79-5
30 suppliers
inquiry