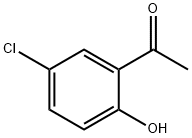
(5-CHLORO-3-METHYL-1-BENZOFURAN-2-YL)(4-CHLOROPHENYL)METHANONE synthesis
- Product Name:(5-CHLORO-3-METHYL-1-BENZOFURAN-2-YL)(4-CHLOROPHENYL)METHANONE
- CAS Number:131866-23-4
- Molecular formula:C16H10Cl2O2
- Molecular Weight:305.16
Yield:131866-23-4 73%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;sodium carbonate in water; for 0.5 h;Reflux;Green chemistry;Rapp-Stoermer Benzofuran Synthesis;
Steps:
Synthesis of 2-aroylbenzofurans
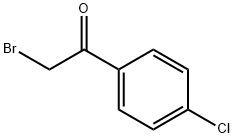
General procedure: Synthesis of 2-(4-bromobenzoyl)-3-methylbenzofuran 3a A mixture of o-hydroxyacetophenone (1.36 g, 0.01 mol), α-bromoketone (2.62 g, 0.01 mol), Na2CO3 (2.12 g, 0.02 mol) and n-Bu4N+I- (0.01 mol) was mixed in a mortar with pestle. This mixture was transferred to a round-bottom flask containing 10.0 ml water and then refluxed on a heating mantle. After completion of the reaction (30 min, TLC), the reaction mixture was cooled at room temperature. The brown-colored solid thus obtained was filtered, dried, and purified by column chromatography on silica gel (100-200 mesh) using petroleum ether/ethyl acetate as eluent mixture (petroleum ether:EtOAc::99:1) to remove the coloured impurities that furnished the pure white product. The other 2-aroylbenzofurans 3b-e and 3a'-e' were also synthesized following a similar procedure by condensing the respective o-hydroxyacetophenones with α-bromoketones.
References:
Jindal, Pooja;Sharma, Geeta;Arora, Rita;Kamboj, Ramesh C. [Research on Chemical Intermediates,2015,vol. 41,# 7,p. 4465 - 4476]