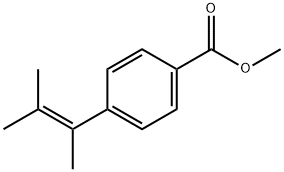
4-(1,2-Dimethyl-1-propen-1-yl)-benzoic Acid Methyl Ester synthesis
- Product Name:4-(1,2-Dimethyl-1-propen-1-yl)-benzoic Acid Methyl Ester
- CAS Number:13399-36-5
- Molecular formula:C13H16O2
- Molecular Weight:204.26
Yield:13399-36-5 87%
Reaction Conditions:
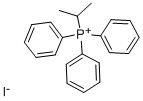
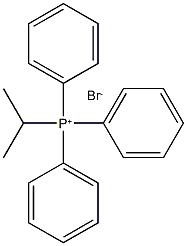
Stage #1: isopropyltriphenylphosphonium iodidewith potassium hexamethylsilazane in toluene at 0; for 0.166667 h;Inert atmosphere;
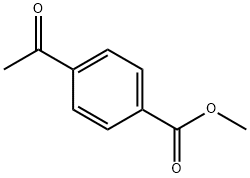
Stage #2: methyl 4-acetylbenzoate in toluene; for 3 h;Reflux;Inert atmosphere;
Steps:
1.1-2 Synthesis Example 1-2. Synthesis of methyl 4-(3-methylbut-2-en-2-yl)benzoate (1-3)
Isopropyl phosphonium iodide salt (242.8 mg, 0.56 mmol, 2 eq.) was dissolved in anhydrous toluene under argon gas, and KHMDS (1.1 mL, 0.56 mmol, 2 eq., 0.5 M toluene solution) was added dropwise at 0 °C. After stirring the red reaction mixture for 10 minutes, methyl-4-acetylbenzoate 1-2 (50 mg, 0.28 mmol, 1 eq) was dissolved in anhydrous toluene (2 mL) and added dropwise at room temperature. The reaction mixture was heated to reflux for 3 hours, cooled to room temperature, diluted with 10% EtOAc/Hexeane (10 mL), and water (5 mL) was added to terminate the reaction. Filtered over CELITE and washed the solid with 10% EtOAc/Hexeane (10 mL). The organic layer of the filtrate was separated and the aqueous layer was extracted with EtOAc (10 mL × 3). The combined organic layers were dried over anhydrous Na2SO4, filtered, and the filtrate was concentrated under reduced pressure. The concentrate was purified by column chromatography (EtOAc:Hexanes = 2:98) to obtain the target compound 1-3 (50 mg, 87%) as a colorless oil.
References:
KR2021/89017,2021,A Location in patent:Paragraph 0088; 0090-0091; 0097-0100
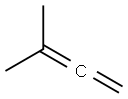
598-25-4
51 suppliers
$66.00/1mL

13399-36-5
2 suppliers
inquiry
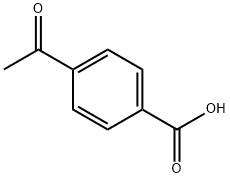
586-89-0
269 suppliers
$10.00/1g

13399-36-5
2 suppliers
inquiry