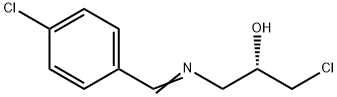
(S)-1-chloro-3-{[(4-chlorophenyl)methylene]amino}propan-2-ol synthesis
- Product Name:(S)-1-chloro-3-{[(4-chlorophenyl)methylene]amino}propan-2-ol
- CAS Number:1345879-87-9
- Molecular formula:C10H11Cl2NO
- Molecular Weight:232.11
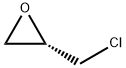
67843-74-7
488 suppliers
$10.00/1g
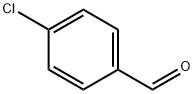
104-88-1
645 suppliers
$5.00/25g
![(S)-1-chloro-3-{[(4-chlorophenyl)methylene]amino}propan-2-ol](/CAS/20180527/GIF/1345879-87-9.gif)
1345879-87-9
18 suppliers
inquiry
Yield:1345879-87-9 75%
Reaction Conditions:
Stage #1: 4-chlorobenzaldehydewith ammonium hydroxide in methanol;
Stage #2: (S)-epichlorohydrin in methanol at 20 - 40;Product distribution / selectivity;
Steps:
28
EXAMPLE 28. (S)(E,Z)-l-(4-Chlorobenzylideneamino)-3-chloropropan-2-ol (18). Alternative procedure. 4-Chlorobenzaldehyde (15.7 gm 112 mmol), methanol (50 ml) and cone NH4OH (10 g, 172 mmol) were added to a 250 ml round bottom flask with football stirring bar. (S)-Epichlorohydrin (10 g, 8.5 ml, 108 mmol) (Atlantic SciTech Group, Inc) (98% purity) was added and the colorless reaction solution was heated at 40 °C (bath temp) for 6 hr with stirring and allowed to stand at room temp overnight. GCMS analysis of the reaction solution indicated complete reaction. The reaction solution was diluted with methylene chloride (100 ml) and brine (75 ml). The organic layer was separated and the aqueous layer extracted with additional methylene chloride (25 ml). The combined organic layer was washed with brine (50 ml), dried (MgS04) and evaporated to a white crystalline solid. (In previous runs initially isolated as an oil that was seeded). The crystals were treated with hexane (30 ml), cooled overnight at -15 °C and collected by filtration, air dried and then dried in a vacuum oven for 3 hours at room temp (19.5 g, 75%); 1H NMR (500 MHz, DMSO- d6) δ 8.34 (s, 1H), 7.78 (d, 2H, J=8.5 Hz) ), 7.52 (d, 2H, J=8.5 Hz), 5.29 (d, 1H, J=5.4 Hz), 3.94(dd, 1H, J= 13 Hz, J= 6.1 Hz), 3.25 (br ddd, 1H, J= 6.5 Hz, J=3.5Hz, J=2.8 Hz), 2.65 (dd, 1H, J= 5 Jz, J= 2.6 Hz), 2.77 (dd, 1H, J= 5Hz, J= 4.1 Hz); GCMS Retention time 10.03 min (m/e 232).
References:
WO2011/137222,2011,A1 Location in patent:Example 28