
3,4,6,7,8,9-hexahydropyrido[3,4-b]indolizin-1(2H)-one synthesis
- Product Name:3,4,6,7,8,9-hexahydropyrido[3,4-b]indolizin-1(2H)-one
- CAS Number:1346672-96-5
- Molecular formula:C11H14N2O
- Molecular Weight:190.24

1346672-95-4
1 suppliers
inquiry
![3,4,6,7,8,9-hexahydropyrido[3,4-b]indolizin-1(2H)-one](/CAS/20150408/GIF/1346672-96-5.gif)
1346672-96-5
6 suppliers
inquiry
Yield:1346672-96-5 220 mg
Reaction Conditions:
with sodium ethanolate in ethanol at 55; for 5 h;Inert atmosphere;
Steps:
112d Example 112d 3,4,6,7,8,9-Hexahydropyrido[3,4-b]indolizin-1(2H)-one 112d
Example 112d
3,4,6,7,8,9-Hexahydropyrido[3,4-b]indolizin-1(2H)-one 112d
A 100-mL single-neck round-bottomed flask equipped with a magnetic stirrer and nitrogen inlet was purged with nitrogen and charged with methyl 3-(2-aminoethyl)-5,6,7,8-tetrahydroindolizine-2-carboxylate hydrogen chloride salt 112c (prepared above, estimated 1.74 mmol, presuming quantitative yield), sodium ethoxide (354 mg, 5.22 mmol) and ethanol (20 mL).
The mixture was stirred at 55 °C for 5 h.
After that time, the reaction mixture was concentrated under reduced pressure and the residue was partitioned between ethyl acetate (200 mL) and water (100 mL).
The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (2 * 100 mL).
The combined organic layers were washed with brine, dried over sodium sulfate and concentrated under reduced pressure.
The residue was purified by column chromatography to afford a 67% yield (220 mg) of 112d as a white solid: mp 195-197 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.76 (s, 1H), 5.89 (s, 1H), 3.78 (t, 2H, J = 6.5 Hz), 3.35 (m, 2H), 2.66 (m, 4H),1.87 (m, 2H), 1.72 (m, 2H); (APCI+) m/z 191.3 (M+H)
References:
EP2773638,2015,B1 Location in patent:Paragraph 0266; 0267

16959-59-4
15 suppliers
inquiry
![3,4,6,7,8,9-hexahydropyrido[3,4-b]indolizin-1(2H)-one](/CAS/20150408/GIF/1346672-96-5.gif)
1346672-96-5
6 suppliers
inquiry
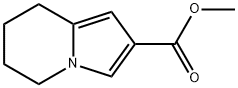
87281-44-5
11 suppliers
inquiry
![3,4,6,7,8,9-hexahydropyrido[3,4-b]indolizin-1(2H)-one](/CAS/20150408/GIF/1346672-96-5.gif)
1346672-96-5
6 suppliers
inquiry

1346672-94-3
1 suppliers
inquiry
![3,4,6,7,8,9-hexahydropyrido[3,4-b]indolizin-1(2H)-one](/CAS/20150408/GIF/1346672-96-5.gif)
1346672-96-5
6 suppliers
inquiry
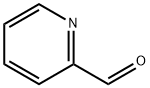
1121-60-4
523 suppliers
$10.60/10gm:
![3,4,6,7,8,9-hexahydropyrido[3,4-b]indolizin-1(2H)-one](/CAS/20150408/GIF/1346672-96-5.gif)
1346672-96-5
6 suppliers
inquiry