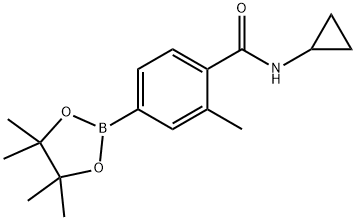
N-Cyclopropyl-2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide synthesis
- Product Name:N-Cyclopropyl-2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide
- CAS Number:1351374-48-5
- Molecular formula:C17H24BNO3
- Molecular Weight:301.19
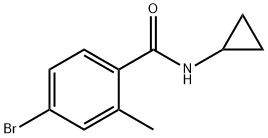
345965-99-3
16 suppliers
inquiry

73183-34-3
586 suppliers
$6.00/5g

1351374-48-5
42 suppliers
$90.00/500mg
Yield:1351374-48-5 94%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in N,N-dimethyl-formamide at 100; for 4 h;Inert atmosphere;
Steps:
N-cyclopropyl-2-methyl-4-f 4, 4, 5, 5-tetramethyl-l , 3, 2-dioxaborolan-2-yl)benzamide mixture of 4-bromo-N-cyclopropyl-2-methylbenzamide
(3.73 g, 14 mmol), Bis(pinacolato)diboron (5.59 g, 22 mmol), anh KOAc (4.29 g, 43 mmol) in DMF (37 mL) was purged with Ar for 10 min at rt. Then PdCl2(dppf) DCM (0.59 g, 5 mol%) was added and the reaction was heated at 100 °C in oil bath for 4 h. After reaction completion the reaction mass was diluted with EtO Ac (200 mL) and H20 (100 mL). The combined layer filtered through celite pad and washed it with little EtO Ac. The aq. layer further extracted with EtOAc (50 mL) and the combined organic layer washed with brine, dried over Na2S04, filtered, and concentrated to give crude oily residue. The crude product was purified by flash chromatography (gradient: EtO Ac/hex 0-100%) to give the title compound as a creamy solid (4.15 g, 94%). NMR (400 MHz, CDCl3) δ ppm 7.66 (s, 1H), 7.63-7.61 (d, J = 7.6 Hz, 1 H), 7.32-7.30 (d, J = 7.6 Hz, 1H), 5.85 (s, 1 H), 2.93- 2.87 (m, 1H), 2.46 (s, 3H), 1.35 (s, 12H), 0.90-0.86 (m, 2H), 0.63-0.59 (m, 2H); MS ESI [M + H]+ 302.2, calcd for [Ci7H24BN03 + H]+ 302.2.
References:
WO2015/70349,2015,A1 Location in patent:Page/Page column 22
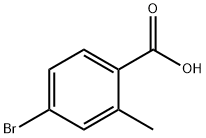
68837-59-2
350 suppliers
$5.00/1g

1351374-48-5
42 suppliers
$90.00/500mg
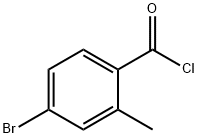
21900-45-8
19 suppliers
inquiry

1351374-48-5
42 suppliers
$90.00/500mg