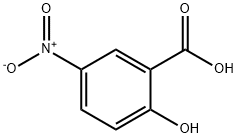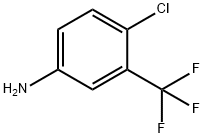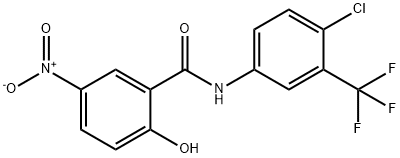
N-[4-Chloro-3-(trifluoroMethyl)phenyl]-2-hydroxy-5-nitrobenzaMide synthesis
- Product Name:N-[4-Chloro-3-(trifluoroMethyl)phenyl]-2-hydroxy-5-nitrobenzaMide
- CAS Number:1352440-75-5
- Molecular formula:C14H8ClF3N2O4
- Molecular Weight:360.67
Yield:1352440-75-5 85%
Reaction Conditions:
with triphenyl phosphite in toluene; for 8 h;Reflux;
Steps:
4.1.1.1. N-Phenyl-2-hydroxy-5-nitrobenzamide (3a)
General procedure: A mixture containing 2-hydroxy-5-nitrobenzoic acid (1.83 g, 10 mmol), aniline (1.21 g, 13 mmol), triphenyl phosphite (3.6 mL) and toluene (30 mL) was strirred under refluxing for 8 h, cooled to room temperature. The resulted solid was collected by filtration under reduced pressure, washed with cooled dichlormethane (5 mL × 2), crystallized from ethanol (20 mL) to produce 2.17 g of 3a as a yellow solid. Yield 84%.
References:
Zuo, Miao;Zheng, Yue-Wen;Lu, She-Min;Li, Yan;Zhang, San-Qi [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 14,p. 4405 - 4412] Location in patent:experimental part