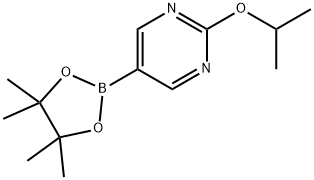
2-Isopropoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine synthesis
- Product Name:2-Isopropoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine
- CAS Number:1355066-82-8
- Molecular formula:C13H21BN2O3
- Molecular Weight:264.13
121487-12-5
27 suppliers
$75.00/250mg

73183-34-3
587 suppliers
$6.00/5g

1355066-82-8
19 suppliers
inquiry
Yield:1355066-82-8 54%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 95 - 105;Inert atmosphere;
Steps:
100
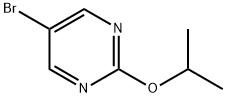
Preparation 1002-lsopropoxy-5-(4,4,5,5- tetramethyl-1 ,3,2-dioxaborolan-2-yl)pyhmidine5-Bromo-2-isoproxypyrinnidine (171 g, 787.8 mmol), bis(pinacolato)diboron (290 g, 1 .14 mol), potassium acetate (237 g, 2.36 mol) and 1 ,1 '-bis(diphenylphosphino)ferrocene palladium dichloride (9.10 g, 12.44 mmol) were mixed under nitrogen at room temperature in dioxane (1 L). The mixture was heated at 95 °C for 30 minutes and then at 105 °C until the reaction was complete. The solution was diluted with water (1 L) and dichloromethane (2 L) and filtered through celite. The layers were separated and the organic layer was washed with water (1 L), dried over sodium sulfate, filtered and evaporated to give an oil. The oil was purified by silica gel chromatography eluting with 0 to 5% EtOAc in hexane to yield the title compound as a white solid (162 g, 54%). 1H NMR (400 MHz, DMSO-d6): δ 1 .30-1 .32 (m, 18H), 5.22-5.28 (m, 1 H), 8.70 (s, 2H). Molecular ion not observed.
References:
WO2012/7869,2012,A2 Location in patent:Page/Page column 106