
methyl 3,4-dihydro-2H-pyrano[2,3-c]pyridine-6-carboxylate synthesis
- Product Name:methyl 3,4-dihydro-2H-pyrano[2,3-c]pyridine-6-carboxylate
- CAS Number:1356163-58-0
- Molecular formula:C10H11NO3
- Molecular Weight:193.2
![methyl {[2-oxo-2-(pent-4-en-1-yloxy)ethyl]carbamoyl}formate](/CAS/20200515/GIF/1356163-76-2.gif)
1356163-76-2
6 suppliers
inquiry
![methyl 3,4-dihydro-2H-pyrano[2,3-c]pyridine-6-carboxylate](/CAS/20180629/GIF/1356163-58-0.gif)
1356163-58-0
25 suppliers
inquiry
Yield:-
Reaction Conditions:
with pyridine;trifluoromethylsulfonic anhydride in dichloromethane at 25; for 6 h;
Steps:
1.b
Example 1; Methyl 3,4-dihydro-2H-pyrano[2,3-c]pyridine-6-carboxylate (b) The Title CompoundMethyl 2-oxo-2-((2-oxo-2-(pent-4-en-l-yloxy)ethyl)amino)acetate (42.5 g, 1 eq) and dichloromethane (DCM) (425 mL) were added to a vessel with stirring followed by the addition of pyridine (17.6 g, 1.2 eq). Tf20 (78.5 g, 1.5 eq) was added over 45 min to the mixture maintaining an internal temperature of ~25 °C. The mixture was stirred for 6 h at which point the reaction was carefully quenched by the addition of 20 wt% aqueous NaOAc (255 mL) to form a biphasic solution. The aqueous layer was extracted with DCM (85 mL). The combined organic layers were washed first with water (127.5 mL) and 10 wt% citric acid solution (170 mL). 6N HC1 (127.5 mL) was added to the mixture to form a biphasic mixture. The two layers were separated and the organic layer was extracted with 6 N HC1 (85 mL). The acidic aqueous layers were combined and DCM (127.5 mL) was added. While maintaining the temperature below 25 °C, 28 wt% aqueous NH4OH was slowly added until the pH of the aqueous layer reached 3-5. The two layers were separated and the aqueous layer was extracted with DCM (85 mL). The combined organic layers were washed with water (85 mL). The organic solution was concentrated under reduced pressure to provide the title compound as an oil which solidified on standing. XH NMR (400 MHz, CDCI3) ?pm 8.17 (s, 1 H), 7.80-7.86 (m, 1 H), 4.26 (t, J=5.19 Hz, 2 H), 3.92 (s, 3 H), 2.79 (t, J=6.44 Hz, 2 H), 1.97-2.09 (m, 2 H); 13C NMR (75 MHz, CDCI3) δ ppm 165.1 1, 154.25, 138.95, 138.64, 130.06, 126.13, 65.55, 51.99, 23.57, 20.67; HRMS (M+Na) m/z, calcd for CioHuN03Na, 216.0637; found, 216.0643.
References:
WO2012/12391,2012,A2 Location in patent:Page/Page column 10
![pent-4-en-1-yl 2-{[(tert-butoxy)carbonyl]amino}acetate](/CAS/20210111/GIF/1356164-00-5.gif)
1356164-00-5
6 suppliers
inquiry
![methyl 3,4-dihydro-2H-pyrano[2,3-c]pyridine-6-carboxylate](/CAS/20180629/GIF/1356163-58-0.gif)
1356163-58-0
25 suppliers
inquiry
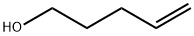
821-09-0
255 suppliers
$21.21/1gm:
![methyl 3,4-dihydro-2H-pyrano[2,3-c]pyridine-6-carboxylate](/CAS/20180629/GIF/1356163-58-0.gif)
1356163-58-0
25 suppliers
inquiry