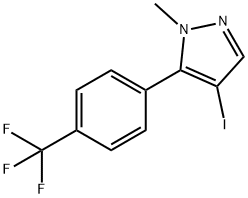
4-iodo-1-methyl-5-(4-(trifluoromethyl)phenyl)-1H-pyrazole synthesis
- Product Name:4-iodo-1-methyl-5-(4-(trifluoromethyl)phenyl)-1H-pyrazole
- CAS Number:1356997-76-6
- Molecular formula:C11H8F3IN2
- Molecular Weight:352.09
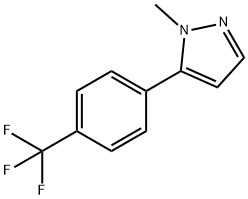
1356997-25-5
6 suppliers
inquiry

1356997-76-6
1 suppliers
inquiry
Yield:1356997-76-6 4.80 g
Reaction Conditions:
with sodium acetate;Iodine monochloride;acetic acid at 20; for 4 h;
Steps:
22 4-Iodo-1-methyl-5-[4-(trifluoromethyl)phenyl]-1H-pyrazole
Production Example 22
4-Iodo-1-methyl-5-[4-(trifluoromethyl)phenyl]-1H-pyrazole
An acetic acid (3.3 mL) solution of iodine monochloride (2.64 g) was added dropwise to an acetic acid (22 mL) solution that contained 1-methyl-5-[4-(trifluoromethyl)phenyl]-1H-pyrazole (3.70 g) and sodium acetate (1.38 g) at a room temperature, and the obtained solution was then stirred for 4 hours.
Thereafter, water (250 mL) was added to the reaction solution, and the obtained solution was then stirred for 30 minutes.
Then, a precipitated solid was collected by filtration, and was then washed with water.
The obtained solid was dissolved in ethyl acetate, and the obtained solution was then washed with water and a saturated sodium hydrogencarbonate aqueous solution.
The organic layer was dried over anhydrous sodium sulfate, and was then concentrated under a reduced pressure.
Thereafter, the residue was purified by column chromatography (silica gel 60N, hexane:ethyl acetate=5:1 to 7:3), so as to obtain the title compound (4.80 g) in the form of a brownish-red oily substance.
1H NMR (600 MHz, CHLOROFORM-d) δ ppm 3.84 (s, 3H) 7.52 (d, J=8.25 Hz, 2H) 7.59 (s, 1H) 7.77 (d, J=7.79 Hz, 2H); MS (ESI pos.) m/z: 353 [M+H]+
References:
US2013/123500,2013,A1 Location in patent:Paragraph 0287; 0288
455-13-0
259 suppliers
$10.00/1g

1356997-76-6
1 suppliers
inquiry