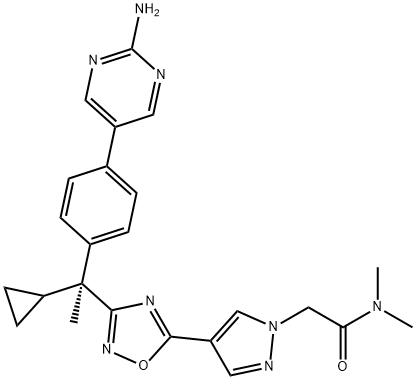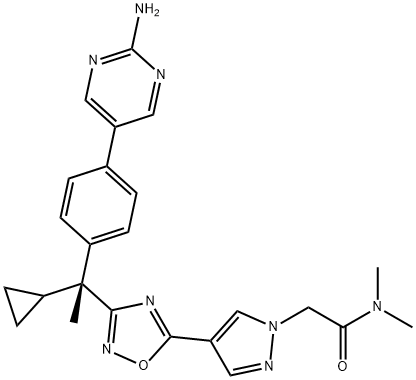
2-[4-[3-[(1R)-1-[4-(2-aminopyrimidin-5-yl)phenyl]-1-cyclopropylethyl]-1,2,4-oxadiazol-5-yl]pyrazol-1-yl]-N,N-dimethylacetamide synthesis
- Product Name:2-[4-[3-[(1R)-1-[4-(2-aminopyrimidin-5-yl)phenyl]-1-cyclopropylethyl]-1,2,4-oxadiazol-5-yl]pyrazol-1-yl]-N,N-dimethylacetamide
- CAS Number:1360550-04-4
- Molecular formula:C24H26N8O2
- Molecular Weight:458.52
Yield:1360550-04-4 32 mg ,1360550-05-5 27 mg
Reaction Conditions:
with Chiralpak AD-H (available form Chiral Technologies, Inc., Exton, Pa.) semi-preparative (250×20 mm) HPLC column in ethanol;n-heptane;diethylamine;Resolution of racemate;
Steps:
Method 18 Synthesis of 2-[4-(3-{(1R)-1-[4-(2-aminopyrimidin-5-yl)phenyl]-1-cyclopropylethyl}-1,2,4-oxadiazol-5-yl)-1H-pyrazol-1-yl]-N,N-dimethylacetamide (Example 115) and 2-[4-(3-{(1S)-1-[4-(2-aminopyrimidin-5-yl)phenyl]-1-cyclopropylethyl}-1,2,4-oxadiazol-5-yl)-1H-pyrazol-1-yl]-N,N-dimethylacetamide (Example 116, Table 1)
Enantiomers 115 and 116 are prepared by resolution of example 59 (100 mg) on a Chiralpak AD-H (available form Chiral Technologies, Inc., Exton, Pa.) semi-preparative (250×20 mm) HPLC column (eluting with 95% EtOH in heptane containing 0.1% diethylamine). The faster eluting enantiomer 115 having a retention time of 35 min and the slower eluting enantiomer 116 having a retention time of 73 min. The eluants are concentrated to provide Example 115 (32 mg) and Example 116 (27 mg
References:
US2013/195879,2013,A1 Location in patent:Paragraph 0421; 0422

1360551-88-7
6 suppliers
inquiry
![2-[4-[3-[(1R)-1-[4-(2-aminopyrimidin-5-yl)phenyl]-1-cyclopropylethyl]-1,2,4-oxadiazol-5-yl]pyrazol-1-yl]-N,N-dimethylacetamide](/CAS/20180601/GIF/1360550-04-4.gif)
1360550-04-4
5 suppliers
inquiry

1360551-90-1
1 suppliers
inquiry
![2-[4-[3-[(1R)-1-[4-(2-aminopyrimidin-5-yl)phenyl]-1-cyclopropylethyl]-1,2,4-oxadiazol-5-yl]pyrazol-1-yl]-N,N-dimethylacetamide](/CAS/20180601/GIF/1360550-04-4.gif)
1360550-04-4
5 suppliers
inquiry

56041-75-9
8 suppliers
inquiry
![2-[4-[3-[(1R)-1-[4-(2-aminopyrimidin-5-yl)phenyl]-1-cyclopropylethyl]-1,2,4-oxadiazol-5-yl]pyrazol-1-yl]-N,N-dimethylacetamide](/CAS/20180601/GIF/1360550-04-4.gif)
1360550-04-4
5 suppliers
inquiry
6952-89-2
134 suppliers
$19.00/1g
![2-[4-[3-[(1R)-1-[4-(2-aminopyrimidin-5-yl)phenyl]-1-cyclopropylethyl]-1,2,4-oxadiazol-5-yl]pyrazol-1-yl]-N,N-dimethylacetamide](/CAS/20180601/GIF/1360550-04-4.gif)
1360550-04-4
5 suppliers
inquiry
![1H-Pyrazole-1-acetamide, 4-[3-[1-[4-(2-amino-5-pyrimidinyl)phenyl]-1-cyclopropylethyl]-1,2,4-oxadiazol-5-yl]-N,N-dimethyl-](/CAS/20210305/GIF/1360549-48-9.gif)