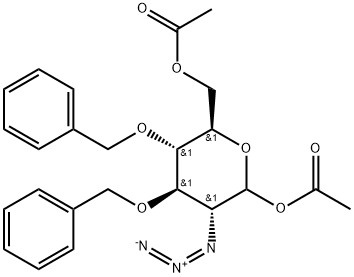
D-Glucopyranose, 2-azido-2-deoxy-3,4-bis-O-(phenylMethyl)-, 1,6-diacetate synthesis
- Product Name:D-Glucopyranose, 2-azido-2-deoxy-3,4-bis-O-(phenylMethyl)-, 1,6-diacetate
- CAS Number:136172-58-2
- Molecular formula:C24H27N3O7
- Molecular Weight:469.49
Yield: 96%
Reaction Conditions:
trifluoroacetic acid in toluene at 18 - 50;
Steps:
b
b) 1,6-di-O-Acetyl-2-azido-3,4-di-O-benzyl-2-deoxy-D-glucopyranose. The reactor is charged with 1650 g of 1,6-anhydro-2-azido-3,4-di-O-benzyl-2-deoxy-D-glucopyranose obtained as described in the above step a) and 6.76 kg of acetic anhydride. Subsequently, 740 g of trifluoroacetic acid is added. The temperature increases from 18 to 22°C. The whole is heated to 50°C and kept at this temperature for 6 hours. On the next day the reaction mixture is concentrated in evaporator, 3 dm3 of toluene is then added to the residue and the mixture is evaporated at the bath temperature 60°C, until condensate ceased to appear in a condenser. This operation is repeated two more times using 1.5 dm3 of toluene each time. The product is cooled down and weighed. The title product (2.2 kg) in the form of a solid mass is obtained with a purity of 92% (HPLC), m.p. 94-100°C (hexane-ethyl acetate 4:1). Yield calculated on a pure product is 96%. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.41-7.25 (m, 10H), 6.23 (d, H, J= 3.5 Hz), 4.94 (d, 1H, J = 10.6Hz), 4.90 (d, 1H, J = 10.6 Hz), 4.87 (d, 1H, J = 10.8 Hz), 4.59 (d, 1H, J = 10.8 Hz), 4.26 (brd, 2H, J = 3.1 Hz), 3.97 (dd, 1H, J3,4= 9.0 Hz, J4,5= 10.2 Hz), 3.93 (dt, 1H, Jt= 3.1 Hz, J4,5= 10.2), 3.63 (dd, 1H, J3,4= 9.0 Hz, J2,3= 10.2 Hz), 3.60 (dd, 1H, J1,2= 3.5 Hz, J2,3= 10.2 Hz), 2.16 (s, 3H), 2.03 (s, 3H). 13C NMR (100 MHz,CDCl3), δ (ppm): 170.54, 168.76, 137.43, 137.20, 128.62, 128.58, 128.22, 128,13, 128.07, 90.37, 80.54, 77.21, 75.69, 75.30, 71.28, 62.73, 62.31, 20.93, 20.77.
References:
Adamed sp. z o.o.;Zaklad Doswiadczalny CHEMIPAN Instytutu Chemii Fizyczneh i Instytutu Chemii Organicznej Polskiej Akademii Nauk EP2374809, 2011, A1 Location in patent:Page/Page column 5
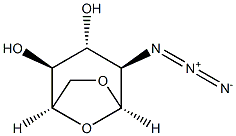
67546-20-7
21 suppliers
inquiry

136172-58-2
24 suppliers
inquiry

100-39-0
429 suppliers
$10.00/10 g

136172-58-2
24 suppliers
inquiry
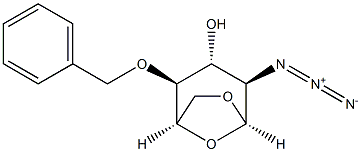
55682-47-8
22 suppliers
inquiry

136172-58-2
24 suppliers
inquiry

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)