
1-Methyl-3-nitro-5-(4,4,5,5-tetraMethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyridin-2-one synthesis
- Product Name:1-Methyl-3-nitro-5-(4,4,5,5-tetraMethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyridin-2-one
- CAS Number:1361941-27-6
- Molecular formula:C12H17BN2O5
- Molecular Weight:280.08
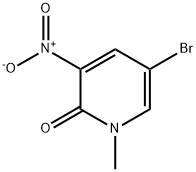
153888-45-0
54 suppliers
$22.00/250mg

73183-34-3
587 suppliers
$6.00/5g
![1-Methyl-3-nitro-5-(4,4,5,5-tetraMethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyridin-2-one](/CAS/20150408/GIF/1361941-27-6.gif)
1361941-27-6
7 suppliers
inquiry
Yield:1361941-27-6 73%
Reaction Conditions:
Stage #1: 5-bromo-1-methyl-3-nitropyridine-2(1H)-one;bis(pinacol)diboranewith potassium acetate in 1,4-dioxane; for 0.5 h;Reflux;Inert atmosphere;
Stage #2: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane at 80; for 2 h;
Steps:
122b
Example 122b l-Methyl-3-nitro-5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)pyridin-2(lH)-one 122bA 3-L three-neck round-bottomed flask equipped with a mechanical stirrer, reflux condenser and thermoregulator was purged with nitrogen and charged with 122a (23.6 g, 102 CGI PHARM6 0WO mmol), 4,4,4' ,4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (32.2 g, 127 mmol), potassium acetate (30.0 g, 306 mmol) and 1,4-dioxane (800 mL). A stream of nitrogen was passed through the resulting suspension for 30 min. Pd(dppf)C12?CH2C12 (4.17 g, 5.10 mmol) was then added, and the reaction was stirred at 80 °C for 2 h. After this time, the mixture was cooled to ambient temperature and evaporated to dryness under reduced pressure at 40 °C. The resulting black solid was charged into a 3-L three-neck round-bottomed flask equipped with a mechanical stirrer and reflux condenser. Toluene (640 mL) and magnesium sulfate (20 g) were added, and the resulting suspension was heated under nitrogen to 90 °C over 15 min. The mixture was filtered hot through a pad of Cellpure P65, and the filter cake was washed with hot toluene (3 x 45 mL). The filtrate was evaporated on a rotary evaporator at 40 °C until a thick suspension formed (weight of the residue was 97 g). This suspension was filtered using the filtrate to transfer the residual material from the walls of the flask. The filter cake was washed with toluene (15 mL) and dried for 2 h under vacuum at 40 °C to afford a 73% yield (20.8 g) of 122b as a yellow solid: ]H NMR (300 MHz, DMSO-i?) δ 8.48 (d, 1H, / = 2.0 Hz), 8.34 (d, 1H, / = 2.0 Hz), 3.60 (s, 3H), 1.30 (s, 12H).
References:
WO2012/31004,2012,A1 Location in patent:Page/Page column 110-111
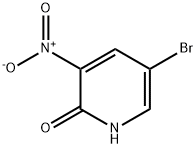
15862-34-7
290 suppliers
$10.00/5g
![1-Methyl-3-nitro-5-(4,4,5,5-tetraMethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyridin-2-one](/CAS/20150408/GIF/1361941-27-6.gif)
1361941-27-6
7 suppliers
inquiry