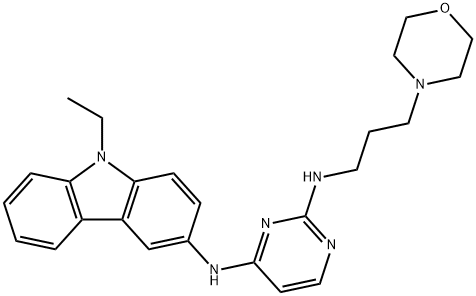
N4-(9-Ethyl-9H-carbazol-3-yl)-N2-(3-Morpholin-4-yl-propyl)-pyriMidine-2,4-diaMine synthesis
- Product Name:N4-(9-Ethyl-9H-carbazol-3-yl)-N2-(3-Morpholin-4-yl-propyl)-pyriMidine-2,4-diaMine
- CAS Number:1380432-32-5
- Molecular formula:C25H30N6O
- Molecular Weight:430.55
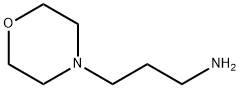
123-00-2

1380432-31-4

1380432-32-5
Synthesis of EHop-016 1H and 13C NMR spectra were recorded on a Bruker 400MHz spectrometer. Mass spectra were obtained on a Hewlett Packard 6890N GC/MS spectrometer. All chemicals were purchased from Sigma Aldrich Chemical Company.The synthesis of EHop-016 (5) was carried out in two steps according to the reaction scheme provided in Figure 1. A process similar to the one described in the literature was carried out as shown in Figure 1(A). (2-Chloro-pyrimidin-4-yl)-(9-ethyl-9H-carbazol-3-yl)-amine 3 was obtained as a pure compound in 53% yield. The product was identified by TLC, NMR and GC/MS. rf = 0.23 (3:1, hexane-ethyl acetate); 1H NMR (DMSO-d6, 400MHz) δ 1.32 (t, J = 6.9Hz, 3H), 4.45 (q, J = 6.6Hz, 2H), 6.72 (s, 1H), 7.20 (t, J = 7.36Hz, 1H ), 7.47 (t, J = 7.30 Hz, 1H), 7.56 (s, 1H), 7.62 (t, J = 8.68 Hz, 1H), 8.11 (t, J = 7.36 Hz, 1H), 8.27 (s, 1H), 10.1 (s, 1H); 13C NMR (DMSO-d6, 100MHz) δ 13.7, 37.0, 109.2, 109.4, 115.0, 118.7, 120.3, 121.3, 121.9, 122.3, 125.9, 129.9, 136.9, 140.0, 156.9, 159.6, 162.4; LRGC-MS m/z (relative percent): [M]+ 276 (100), [M-Cl]+ 241 (40), [M-C5H5N3Cl]+ 134 (26). N4-(9-ethyl-9H-carbazol-3-yl)-N2-(3-morpholin-4-yl-propyl)-pyrimidine-2,4-diamine 5 (EHop-016) was obtained as a pure compound in 93% yield. The product was identified as essentially pure by TLC and NMR: Rf = 0.34 (9:1, CH2Cl2-MeOH); 1H NMR (DMSO-d6, 400 MHz) δ 1.31 (t, J = 7.0 Hz, 3H), 1.73 (m, 2H), 2.32 (m, 2H), 2.34 (t, J = 6.89 Hz, 8H), 3.52 (m, 2H), 4.42 (q, J = 7.0 Hz, 2H), 5.98 (d, J = 5.7 Hz, 1H), 6.69 (t, J = 5.3 Hz, 1H), 7.16 (t, J = 7.4 Hz, 1H), 7.43 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 9.0 Hz, 4H), 7.81 ( d, J = 5.4 Hz, 1H), 8.10 (s, 1H), 8.66 (s, 1H), 9.1 (s, 1H); 13C NMR (DMSO-d6, 100 MHz) δ 13.7, 26.2, 36.9, 53.4, 56.3, 66.2, 108.9, 109.0, 118.2, 119.7, 120.2, 122.0, 122.2, 125.6, 132.5, 135.5, 139.9, 159.8, 160.9, 162.1.

123-00-2
270 suppliers
$6.00/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1380432-32-5
114 suppliers
$39.00/5mg
Yield: 93%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in iso-butanol at 120; for 0.5 h;Microwave irradiation;
Steps:
Synthesis of EHop-016
4Synthesis of EHop-016 [0031] 1H and 13C NMR spectra were recorded on a Bruker 400 MHz Spectrometer. Mass spectra were obtained on a Hewlett Packard 6890N GC/MS Spectrometer. All chemicals were purchased from Sigma Aldrich Chemical Company. The synthesis of EHop-016 (5) was performed in two steps according to the reaction scheme provided in FIG. 1(A), and carried out analogous to the procedure described in (58). (2-Chloro-pyrimidin-4-yl)-(9-ethyl-9H-carbazol-3-yl)-amine 3 was obtained as a pure compound in a yield of 53%. The product was identified with TLC, NMR and GC/MS. Rf=0.23 (3:1, Hexane-Ethyl Acetate); 1H NMR (DMSO-d6, 400 MHz) δ 1.32 (t, J=6.9 Hz, 3H), 4.45 (q, J=6.6 Hz, 2H), 6.72 (s, 1H), 7.20 (t, J=7.36 Hz, 1H), 7.47 (t, J=7.30 Hz, 1H), 7.56 (s, 1H), 7.62 (t, J=8.68 Hz, 1H), 8.11 (t, J=7.36 Hz, 1H), 8.27 (s, 1H), 10.1 (s, 1H); 13C (DMSO-d6, 100 MHz) δ 13.7, 37.0, 109.2, 109.4, 115.0, 118.7, 120.3, 121.3, 121.9, 122.3, 125.9, 129.9, 136.9, 140.0, 156.9, 159.6, 162.4; LRGC-MS m/z (rel %):[M]+ 276 (100), [M-Cl]+ 241 (40), [M-C5H5N3Cl]+ 134 (26). N4-(9-Ethyl-9H-carbazol-3-yl)-N2-(3-morpholin-4-yl-propyl)-pyrimidine-2,4-diamine 5 (EHop-016) was obtained as a pure compound in a yield of 93%. The product was identified to be essentially pure by TLC and NMR: Rf=0.34 (9:1, CH2Cl2-MeOH); 1H NMR (DMSO-d6, 400 MHz) δ 1.31 (t, J=7.0 Hz, 3H), 1.73 (m, 2H), 2.32 (m, 2H), 2.34 (t, J=6.89 Hz, 8H), 3.52 (m, 2H), 4.42 (q, J=7.0 Hz, 2H), 5.98 (d, J=5.7 Hz, 1H), 6.69 (t, J=5.3 Hz, 1H), 7.16 (t, J=7.4, 1H), 7.43 (t, J=7.2 Hz, 1H), 7.53 (t, J=9.0 Hz, 4H), 7.81 (d, J=5.4 Hz, 1H), 8.10 (s, 1H), 8.66 (s, 1H), 9.1 (s, 1H); 13C (DMSO-d6, 100 MHz) δ 13.7, 26.2, 36.9, 53.4, 56.3, 66.2, 108.9, 109.0, 118.2, 119.7, 120.2, 122.0, 122.2, 125.6, 132.5, 135.5, 139.9, 159.8, 160.9, 162.1.
References:
UNIVERSITY OF PUERTO RICO;Hernandez, Eliud;Vlaar, Cornelis;Dharmawardhane, Suranganie US2013/172552, 2013, A1 Location in patent:Paragraph 0031
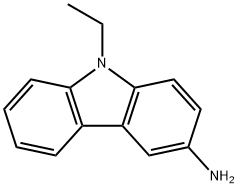
132-32-1
255 suppliers
$25.39/1g

1380432-32-5
114 suppliers
$39.00/5mg