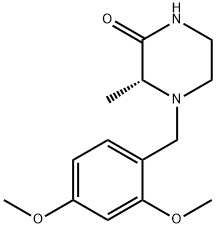
(R)-4-(2,4-diMethoxybenzyl)-3-Methylpiperazin-2-one synthesis
- Product Name:(R)-4-(2,4-diMethoxybenzyl)-3-Methylpiperazin-2-one
- CAS Number:1383146-20-0
- Molecular formula:C14H20N2O3
- Molecular Weight:264.32

922178-61-8
134 suppliers
$63.00/100mg
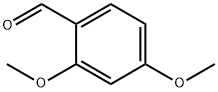
613-45-6
500 suppliers
$10.00/5g

1383146-20-0
102 suppliers
$90.00/50mg
Yield: 98%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in acetonitrile at 20;Inert atmosphere;
Steps:
H General Method H
General procedure: General Method H General Method A is the procedure used for the synthesis of (R)-4-(2,4-dimethoxybenzyl)-3-methylpiperazin-2-one (R)-C(cf. Scheme 30).In a round-bottom flask, were sequentially introduced (R)-3-methylpiperazin-2-one (R)-A (725 mg, 6.35 mmol, leq.), 2,4-dimethoxybenzaldehydeB (1.16 g, 6.99 mmol, 1.1 eq.), acetic acid (545 μΐ, 9.53 mmol, 1.5 eq.) and sodium triacetoxyborohydride (1.88 g, 8.89 mmol, 1.4 eq.) in commercial anhydrous acetonitrile (65 mL), at RT, under N2 atmosphere. The reaction was stirred at RT overnight. The reaction mixture was quenched carefully at 0 °C with saturated NaHC03 solution (100 mL) until no more bubbling was observed. Aqueous and organic layers were separated. The aqueous layer was extracted with EtOAc (3 x 100 mL) and the combined organic layers were washed with brine, dried over MgS04' filtered, and concentrated under reduced pressure to afford the title compound as yellow oil. The crude compound was then purified on silica gel (DCM/MeOH: 98/2 to 95/5) to afford the desired product (R)-C as a viscous pale yellow oil. Yield: 1.65 g, 98 %. LCMS: P = 100 , retention time = 1.6 min, (M+H)+: 265; chiral HPLC retention time = 41.5 min, ee > 99 ; 1H-NMR (CDC13): δ 7.23 (d, J= 8.9, IH), 6.49 (d, J= 8.9, IH), 6.46 (s, IH), 6.29 (br, IH), 3.81 (s, 3H), 3.80 (s, 3H), 3.78 (d, JAB= 15.0, IH), 3.49 (d, JAB= 15.0, IH), 3.27 (m, 2H), 3.19 (m, IH), 2.95 (m, IH), 2.48 (m, IH), 1.48 (d, J= 6.8, 3H).
References:
EUROSCREEN S.A.;HOVEYDA, Hamid;DUTHEUIL, Guillaume;FRASER, Graeme;ROY, Marie-Odile;EL BOUSMAQUI, Mohamed;BATT, Frédéric WO2013/50424, 2013, A1 Location in patent:Page/Page column 136-137