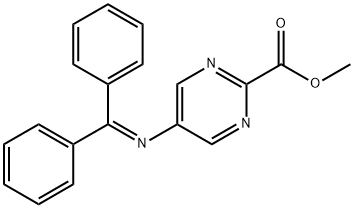
methyl 5-(diphenylmethyleneamino)pyrimidine-2-carboxylate synthesis
- Product Name:methyl 5-(diphenylmethyleneamino)pyrimidine-2-carboxylate
- CAS Number:1383802-11-6
- Molecular formula:C19H15N3O2
- Molecular Weight:317.34
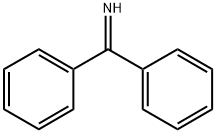
1013-88-3
393 suppliers
$10.00/5g
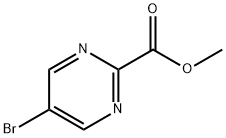
89581-38-4
167 suppliers
$7.00/100mg

1383802-11-6
2 suppliers
inquiry
Yield:1383802-11-6 57%
Reaction Conditions:
with palladium(II) acetate trimer;Cs2CO3;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in toluene at 100; for 5 h;Inert atmosphere;
Steps:
methyl 5-((diphenylmethylene)amino)pyrimidine-2-carboxylate (72)
A suspension of methyl 5-bromopyrimidine-2-carboxylate (70, 2.0 g, 9.22 mmol), diphenylmethanimine (1.67 g, 9.22 mmol), BINAP (0.57 g, 0.92 mmol) and caesium carbonate (3.90 g, 12.0 mmol) in Toluene (20 mL) was stirred under nitrogen at rt. This was degassed with nitrogen for 15 min before adding palladium (II) acetate (0.21 g, 0.92 mmol) in one charge. The reaction was stirred at 100°C for 5 h. The reaction mixture was diluted with EtOAc (40 mL) and washed with water (35 mL). The aqueous layer was further extracted with EtOAc (2×35 mL). The combined organic layer was dried over anh. Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (100g silica, 35% EtOAc in pet. ether) to afford the title compound 72 as yellow solid (2.0 g, 5.29 mmol, 57%). MS (ES+): m/z (%) 318 (100) [M+H]+.
References:
Cantizani, Juan;Cotillo, Ignacio;De Rycker, Manu;Dodd, Peter G.;Ferguson, Liam;Gilbert, Ian H.;Manzano, Pilar;Marco, Maria;McGonagle, Kate;Miles, Tim;Naylor, Claire;Osuna-Cabello, Maria;Paterson, Christy;Pinto, Erika G.;Read, Kevin D.;Riley, Jennifer;Scullion, Paul;Shishikura, Yoko;Simeons, Frederick;Stojanovski, Laste;Svensen, Nina;Tarver, Gary J.;Thomas, John;Thomas, Michael G.;Wyatt, Paul G. [European Journal of Medicinal Chemistry,2022,vol. 238,art. no. 114421] Location in patent:supporting information