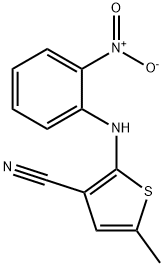
5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile synthesis
- Product Name:5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile
- CAS Number:138564-59-7
- Molecular formula:C12H9N3O2S
- Molecular Weight:259.28
1493-27-2
138564-58-6
![5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile](/CAS/GIF/138564-59-7.gif)
138564-59-7
A solution of 1-fluoro-2-nitrobenzene (34.5 g, 244 mmol) and 2-amino-3-cyano-5-methylthiophene (33.1 g, 240 mmol) in anhydrous THF (160 mL) was added slowly and dropwise to a vigorously stirred anhydrous tetrahydrofuran (THF, 100 mL) suspension of sodium hydride (NaH, 13.5 g, 60% dispersed in mineral oil, 336 mmol), cooled in an ice bath, and under nitrogen protection. mmol) in a solution of anhydrous THF (160 mL). After dropwise addition, the reaction mixture was stirred at room temperature for 10 hours. Subsequently, additional sodium hydride (NaH, 1.53 g, 95%, 72 mmol) was slowly added to the reaction mixture and stirring was continued for 8 hours at room temperature. Upon completion of the reaction, the mixture was poured into crushed ice and the pH was adjusted to 8 with saturated ammonium chloride (NH4Cl) solution. the precipitate was collected by filtration and dried to give the crude product. The crude product was purified by silica gel column chromatography (eluent: 10% ethyl acetate/hexane) to afford 2-(2-nitroanilino)-3-cyano-5-methylthiophene (47.9 g, 77% yield) as a dark solid. Thin layer chromatography (TLC) Rf value was 0.62 (25% ethyl acetate/hexane). The melting point is 105-107°C. Nuclear magnetic resonance hydrogen spectrum (1H NMR, CDCl3): δ 2.47 (d, J = 1.0 Hz, 3H, CH3), 6.78 (q, J = 1.0 Hz, 1H, Ar-H), 6.95 (ddd, J = 1.0, 7.0, 8.5 Hz, 1H, Ph-H), 7.18 (dd, J = 1.0, 8.5 Hz, 1H, Ph-H) , 7.50 (dd, J = 1.5, 7.0, 8.5 Hz, 1H, Ph-H), 8.24 (dd, J = 1.5, 8.5 Hz, 1H, Ph-H), 9.61 (s, 1H, NH). Mass spectrum (MS, ESI): m/z 258 ([M-H]? , 100%).

1493-27-2
507 suppliers
$5.00/10g

138564-58-6
363 suppliers
$8.00/1g
![5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile](/CAS/GIF/138564-59-7.gif)
138564-59-7
312 suppliers
$6.00/1g
Yield:138564-59-7 77%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil at 20; for 18 h;Inert atmosphere;Cooling with ice;
Steps:
5-Methyl-2-((2-nitrophenyl)amino)thiophene-3-carbonitrile (2)
A solution of 1-fluoro-2-nitrobenzene (34.5 g, 244 mmol) and compound 1 (33.1 g, 240 mmol) in dry THF (160 mL) was added dropwise under N2 atmosphere to a vigorously stirred suspension of NaH (13.5 g, 60% dispersion in oil, 336 mmol) in dry THF (100 mL) in an ice bath. After the reaction mixture was stirred at room temperature (RT) for 10 h, additional NaH (1.53 g, 95%, 72 mmol) was slowly added to above reaction mixture. The mixture was stirred for another 8 h at rt, and poured into cracked ice, adjusted pH value to 8 with saturated NH4Cl. The resulting precipitate was filtered, and dried; and the crude product was purified by column chromatography (10% EtOAc/hexanes) on silica gel to obtain 2 (47.9 g, 77%) as a darkened solid; Rf = 0.62 (25% EtOAc/hexanes); mp 105-107 °C. 1H NMR (CDCl3): δ 2.47 (d, J = 1.0 Hz, 3H, CH3), 6.78 (q, J = 1.0 Hz, 1H, Ar-H), 6.95 (ddd, J = 1.0, 7.0, 8.5 Hz, 1H, Ph-H), 7.18 (dd, J = 1.0, 8.5 Hz, 1H, Ph-H), 7.50 (ddd, J = 1.5, 7.0, 8.5 Hz, 1H, Ph-H), 8.24 (dd, J = 1.5, 8.5 Hz, 1H, Ph-H), 9.61 (s, 1H, NH). MS (ESI): 258 ([M-H]-, 100%).
References:
Gao, Mingzhang;Shi, Zenas;Wang, Min;Zheng, Qi-Huang [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 7,p. 1953 - 1956]
609-73-4
18 suppliers
$10.00/5g

138564-58-6
363 suppliers
$8.00/1g
![5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile](/CAS/GIF/138564-59-7.gif)
138564-59-7
312 suppliers
$6.00/1g
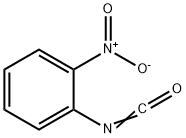
3320-86-3
91 suppliers
$42.10/5g
![5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile](/CAS/GIF/138564-59-7.gif)
138564-59-7
312 suppliers
$6.00/1g