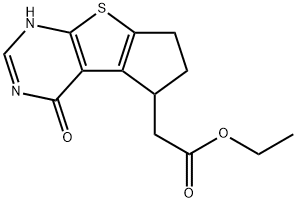
ethyl 4-hydroxy-6,7-dihydro-5H-cyclopenta[4,5]thieno[2,3-d]pyrimidine-5-carboxylate synthesis
- Product Name:ethyl 4-hydroxy-6,7-dihydro-5H-cyclopenta[4,5]thieno[2,3-d]pyrimidine-5-carboxylate
- CAS Number:1388893-76-2
- Molecular formula:C13H14N2O3S
- Molecular Weight:278.33
Yield:1388893-76-2 57%
Reaction Conditions:
at 180; for 8 h;
Steps:
2 Step 2-Ethyl 2-(1-hydroxy-7,8-dihydro-6H-cyclopenta[3,4]thieno[1,3-c]pyrimidin-8-yl)acetate
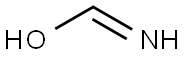
A mixture of ethyl 2-amino-4-(2-ethoxy-2-oxo-ethyl)-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxylate (28.0 g, 94.1 mmol) and formamide (158 g, 3.51 mol) was stirred at 180° C. for 8 h. On completion, the reaction mixture was cooled to room temperature and then quenched with water/ice (200 mL). The mixture was extracted with ethyl acetate (3×100 mL) and the combined organic layer was dried over anhydrous Na2SO4, filtered and concentrated in vacuo to give the title compound (15.0 g, 57% yield) as a yellow brown solid. 1H NMR (400 MHz, DMSO-d6) δ 12.49 (s, 1H), 8.03 (s, 1H), 4.05 (q, J=7.2 Hz, 2H), 3.66-3.52 (m, 1H), 3.28 (dd, J=3.6, 16 Hz, 1H), 3.08-2.81 (m, 2H), 2.74-2.59 (m, 1H), 2.40-2.30 (m, 1H), 2.18-1.99 (m, 1H), 1.15 (t, J=7.2 Hz, 3H); LC-MS (ESI+) m/z 279.0 (M+H)+.
References:
US2019/192668,2019,A1 Location in patent:Paragraph 2499; 2501
20826-94-2
174 suppliers
$26.00/1g
![ethyl 4-hydroxy-6,7-dihydro-5H-cyclopenta[4,5]thieno[2,3-d]pyrimidine-5-carboxylate](/CAS/GIF/1388893-76-2.gif)
1388893-76-2
9 suppliers
inquiry
![diethyl 2-amino-5,6-dihydro-4H-cyclopenta[b]thiophene-3,4-dicarboxylate](/CAS/GIF/1029689-54-0.gif)