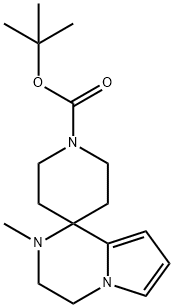
Spiro[piperidine-4,1'(2'H)-pyrrolo[1,2-a]pyrazine]-1-carboxylic acid, 3',4'-dihydro-2'-methyl-, 1,1-dimethylethyl ester synthesis
- Product Name:Spiro[piperidine-4,1'(2'H)-pyrrolo[1,2-a]pyrazine]-1-carboxylic acid, 3',4'-dihydro-2'-methyl-, 1,1-dimethylethyl ester
- CAS Number:1392466-04-4
- Molecular formula:C17H27N3O2
- Molecular Weight:305.42
Yield:1392466-04-4 78%
Reaction Conditions:
with toluene-4-sulfonic acid in ethanol at 70; for 4 h;
Steps:
3 Step 3:
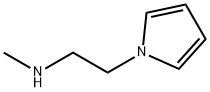
N-Methyl-2-pyrrol-1-yl-ethanamine (2.19 g, 17.64 mmol), tert-butyl 4-oxopiperidine-1-carboxylate (3.51 g, 17.64 mmol), and pTsOH.H2O (0.334 g, 1.76 mmol) were combined in ethanol (87.60 mL) and heated at 70° C. for 4 h. The reaction was concentrated and the residue was dissolved in dichloromethane. The organics were washed with a saturated NaHCO3 solution and brine. The organics were dried over sodium sulfate and evaporated. The crude material was purified by silica gel chromatography eluting with 0-10% methanol in dichloromethane with 2% triethylamine to give tert-butyl 2-methylspiro[3,4-dihydropyrrolo[1,2-a]pyrazine-1,4'-piperidine]-1'-carbo- xylate (4.2 g, 78%). ESI-MS m/z calc. 305.4. found 306.3 (M+1)+; Retention time: 0.97 minutes (3 min run). 1H NMR (400 MHz, CDCl3) δ 6.55-6.52 (m, 1H), 6.15-6.11 (m, 1H), 5.92-5.89 (m, 1H), 3.92 (t, J=6.0 Hz, 2H), 3.91-3.75 (m, 2H), 3.29 (t, J=6.0 Hz, 2H), 3.26-3.12 (m, 2H), 2.36 (s, 3H), 2.10-1.99 (m, 2H), 1.83-1.69 (m, 2H), 1.47 (s, 9H).
References:
US2012/196869,2012,A1 Location in patent:Paragraph 0197; 0198
77200-24-9
72 suppliers
$45.00/50mg
![Spiro[piperidine-4,1'(2'H)-pyrrolo[1,2-a]pyrazine]-1-carboxylic acid, 3',4'-dihydro-2'-methyl-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1392466-04-4.gif)
1392466-04-4
0 suppliers
inquiry