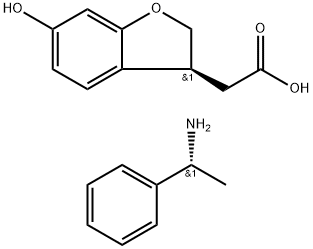
(R)-1-phenylethanamine (S)-2-(6-hydroxy-2,3-dihydrobenzofuran-3-yl)acetate synthesis
- Product Name:(R)-1-phenylethanamine (S)-2-(6-hydroxy-2,3-dihydrobenzofuran-3-yl)acetate
- CAS Number:1394138-46-5
- Molecular formula:C18H21NO4
- Molecular Weight:315.37

1394138-45-4
3 suppliers
inquiry

1394138-46-5
13 suppliers
inquiry
Yield:99.7 % de
Reaction Conditions:
with hydrogen;dichloro [(+)-1,2-bis((2R,5R)-2,5-diisopropylphosphorano)benzene]ruthenium (II)-N,N-dimethylformamide complex in methanol at 35; under 8250.83 Torr; for 26 h;
Steps:
2
Example 2Synthesis of [ (3S) -6-hydroxy-2, 3-dihydro-l-benzofuran-3- yl] acetic acid (1R) -1-phenylethylamine salt; [0387]IA solution of (6-hydroxy-l-benzofuran-3-yl) acetic acid (1R) -1-phenylethylamine salt (8.16 g) and dichloro [ (+) -1, 2- bis ( (2R, 5R) -2, 5-diisopropylphosphorano) benzene] ruthenium (II) -5 N, N-dimethylformamide complex (1.3 mg) in dehydrated methanol (50 ml) was stirred at 35°C for 26 hr under a hydrogenatmosphere (1.1 MPa) . After concentration, the residue was dissolved in a mixed solvent of methanol (25.0 mL) -water (3.7 mL) at around 55°C. After adding toluene (225 mL) at the same0 temperature, the mixture was stirred at 25°C for 20 hr and at 0°C for 1 hr. The precipitated crystals were collected by filtration, washed with toluene (25 mL) , and dried at 50°C under reduced pressure to give a crude product (6.65 g) . The crude product was subjected to a similar recrystallization5 method using a mixed solvent of methanol (20.0 mL) -water (3.0 mL) and diisopropyl ether (180 mL) to give a crude product (6.16 g) . The crude product was subjected to a similarrecrystallization method using a mixed solvent of methanol (18.5 mL) -water (2.8 mL) and diisopropyl ether (166 mL) to0 give the title compound (5.81 g) as white crystals. 99.7%de.XH NMR (500 MHz, DMSO-d6) : δ 1.32 (d, 3H, J = 7.0 Hz), 2.32 (dd, 1H, J = 15.9, 9.3 Hz), 2.56 (dd, 1H, J = 15.9, 5.7 Hz), 3.54- 3.67 (m, 1H) , 4.05-4.17 (m, 2H) , 4.63 (t, 1H, J = 9.0 Hz), 6.15 (1H, d, J = 2.2 Hz), 6.22 (dd, 1H, J = 8.2, 2.2 Hz), 6.97(dd, 1H, J = 8.0, 0.8 Hz), 7.20-7.29 (m, 1H) , 7.29-7.38 (m,2H), 7.38-7.44 (m, 2H) . (protons derived from NH, OH and COOH were not detected)(high performance liquid chromatography conditions)column: CHIRALPAK AD-H (manufactured by DAICEL CHEMICALINDUSTRIES, LTD.)mobile phase: normal hexane/ethanol/trifluoroacetic acid(volume ratio: 90/10/0.1)flow rate: 1.0 mL/mindetection: UV 220 nmtemperature: 30°C
References:
WO2012/111849,2012,A1 Location in patent:Page/Page column 144-145
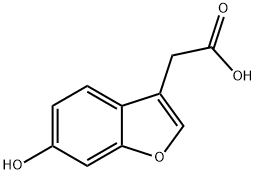
69716-04-7
111 suppliers
$19.00/1g
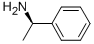
3886-69-9
589 suppliers
$14.00/25G

1394138-46-5
13 suppliers
inquiry
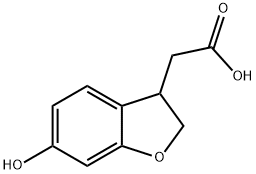
1000414-37-8
34 suppliers
$65.00/100mg
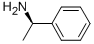
3886-69-9
589 suppliers
$14.00/25G

1394138-46-5
13 suppliers
inquiry
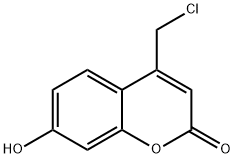
25392-41-0
108 suppliers
$45.00/50mg

1394138-46-5
13 suppliers
inquiry
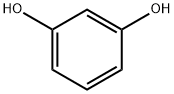
108-46-3
794 suppliers
$10.00/10g

1394138-46-5
13 suppliers
inquiry