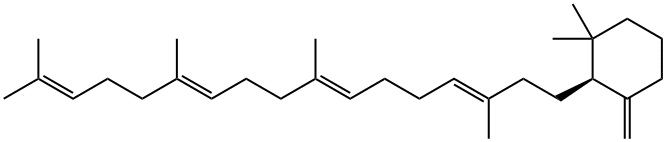
Cyclohexane, 1,1-dimethyl-3-methylene-2-[(3E,7E,11E)-3,8,12,16-tetramethyl-3,7,11,15-heptadecatetraenyl]-, (2S)- (9CI) synthesis
- Product Name:Cyclohexane, 1,1-dimethyl-3-methylene-2-[(3E,7E,11E)-3,8,12,16-tetramethyl-3,7,11,15-heptadecatetraenyl]-, (2S)- (9CI)
- CAS Number:140844-96-8
- Molecular formula:C30H50
- Molecular Weight:410.72
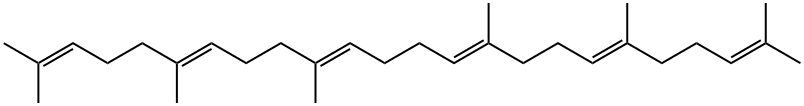
111-02-4
216 suppliers
$15.68/25ML
![Cyclohexane, 1,1-dimethyl-3-methylene-2-[(3E,7E,11E)-3,8,12,16-tetramethyl-3,7,11,15-heptadecatetraenyl]-, (2S)- (9CI)](/CAS/20210111/GIF/140844-96-8.gif)
140844-96-8
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with Triton X-100;cell-free extract A of mutant squalene-hopene cyclase in aq. buffer at 60; for 16 h;
Steps:
1 Synthesis of 3-deoxyachilleol A
Squalene (50 mg) was mixed with Triton X-100 (1 g) to be solubilized, and a buffer A (5 mL) was added theretoto prepare squalene liquid. The whole of the squalene liquid was added to the cell-free extract A to obtain a reactionliquid, followed by incubation at 60°C for 16 hours. In the reaction liquid, the mole ratio (substrate /enzyme) of squalene(substrate) to the mutant squalene-hopene cyclase (enzyme) was about 1,000.After the incubation, ethanol solution containing 15% by mass of potassium hydroxide (KOH/MeOH, 450 mL)was added to the reaction liquid to stop an enzyme reaction. Thereafter, n-hexane (750 mL) was added to the reactionliquid, and the reaction product was extracted three times. The obtained extract was subjected to silica gel columnchromatography (solvent: n-hexane) to obtain pure 3-deoxyachilleol A (42.2 mg). The structure of 3-deoxyachilleol Awas confirmed by gas chromatography-mass spectrometer (GC-MS) and nuclear magnetic resonance apparatus (NMR).
References:
EP3042960,2016,A1 Location in patent:Paragraph 0101; 0103; 0104